
Case 1
A 39-year-old female is transferred to your emergency department from an outpatient same-day surgery center. The patient reportedly developed tachycardia and tachypnea while in the postoperative suite approximately 30 minutes after undergoing general anesthesia for a colonoscopy. She was sent to your emergency department for evaluation, where she was found to be hyperthermic with a rectal temperature of 106.5ºF. You notice a rising end-tidal CO2 despite no change in ventilation and severe masseter muscle spasm.
Explore This Issue
ACEP Now: Vol 35 – No 12 – December 2016Case 2
A 73-year-old male with a past medical history of Parkinson’s disease presents to the emergency department from home with fever and altered mental status. His vital signs are blood pressure 98/59, pulse 102, respiration 15, and temperature 102.6ºF. On exam, you recognize “lead pipe” rigidity and bradykinesia. A septic workup is performed, but there is no obvious source of infection. His wife reports he stopped taking his “Parkinson’s medication” a few days ago.
Case 3
A 27-year-old male with a past medical history of depression, currently treated with fluoxetine, is brought into the emergency department by his girlfriend for altered mental status. He is febrile with a temperature of 102.2ºF, but the rest of his vital signs are within normal limits. He is agitated, is diaphoretic, and has lower extremity clonus. His girlfriend shows you a bottle of dextromethorphan and reports the patient started taking it yesterday for a cough.
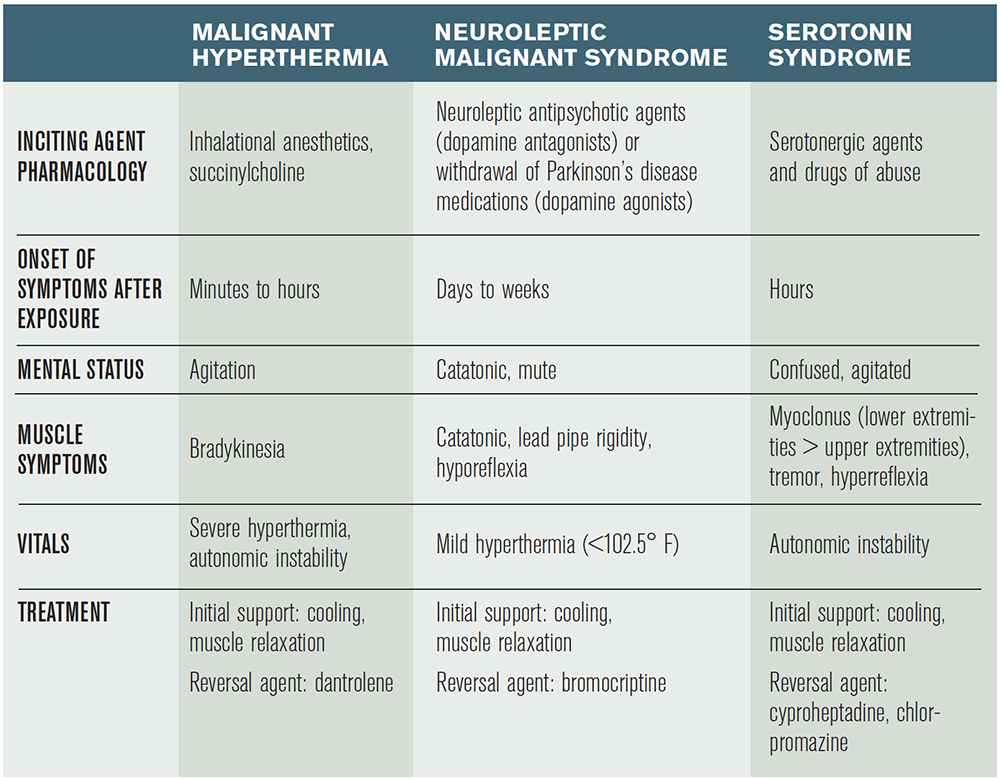
The cases above describe three different hyperthermic disorders: malignant hyperthermia, neuroleptic malignant syndrome (NMS), and serotonin syndrome. These syndromes are often confused with one another when being considered in the diagnosis of a hot and stiff patient presenting to the emergency department (see Table 1).
Malignant Hyperthermia
Malignant hyperthermia is a hypermetabolic syndrome of skeletal muscle, which can occur in individuals with inherited defects of their ryanodine L-type calcium channels. These calcium-release channels are located in the sarcoplasmic reticulum of skeletal muscle and dump out large quantities of calcium ions when stimulated by exposure to certain agents.1 The increase in intracellular calcium levels in skeletal muscle causes increased muscle contraction and increased consumption of adenosine triphosphate, releasing excessive heat, resulting in hyperthermia. The most common agents responsible for causing malignant hyperthermia are certain volatile gas anesthetics (halothane, sevoflurane, desflurane) and succinylcholine. There have also been case reports of excessive exercise and heat leading to the condition.2
The onset of symptoms is relatively rapid, typically presenting within minutes to hours. Diagnosis can occasionally be delayed until the postoperative period in patients undergoing general anesthesia, as described in the patient in Case 1. The most common presenting symptom is unexplained hypercarbia (a rising end-tidal CO2 despite no change in ventilation), followed by hyperthermia, sinus tachycardia, and masseter muscle spasm.3 Often, these patients will develop rhabdomyolysis, which can lead to hyperkalemia, worsening acidosis, and even cerebral edema. The management of patients with presumed malignant hyperthermia begins with benzodiazepines for muscle relaxation, aggressive cooling measures, and early correction of hyperkalemia. The reversal agent for malignant hyperthermia is dantrolene (initial dose is 2.5 mg/kg IV), a hydantoin derivative that functions by inhibiting calcium release from the sarcoplasmic reticulum by antagonizing ryanodine receptors. Dantrolene can cause diaphragmatic weakness. Close monitoring of respiratory status is recommended. For patients who are untreated, mortality has been reported to be as high as 70 percent versus about 7 percent for those who receive proper management.4 The hotline run by the Malignant Hyperthermia Association of the United States (800-644-9737) can be consulted for assistance in treatment.
Neuroleptic Malignant Syndrome
Case 2 describes the presentation of NMS, an idiosyncratic reaction secondary to an overall dopamine deficit caused by either excessive dopamine antagonism from certain antipsychotic agents (eg, haloperidol, olanzapine, quetiapine, risperidone) or from withdrawal of dopamine agonists (eg, Parkinson’s patients who abruptly stop their dopamine agonist medications).5 The dopamine deficit in the hypothalamus and basal ganglia leads to hyperthermia as a result of extrapyramidal side effects like sustained muscle contraction and an elevated temperature set point. There is also a theorized removal of tonic inhibition from the sympathetic nervous system, which results in characteristic autonomic instability.6
“Those individuals who were taking an antipsychotic medication as a cause for their NMS can be treated with bromocriptine, a dopamine agonist used to overcome dopaminergic blockade. There are case reports for the use of dantrolene in NMS. However, this remains controversial.”
The onset of symptoms for NMS is longer than the other hyperthermic disorders, typically presenting within days to weeks of starting or increasing the dose of a dopamine antagonist or withholding a dopamine agonist. The classic finding on physical exam is a catatonic patient with lead pipe rigidity and bradykinesia. Other symptoms include Parkinsonian-like manifestations including dystonia, akathisias, and resting tremors. Management again begins in the emergency department with active cooling and benzodiazepines for muscle relaxation. Patients with NMS are also susceptible to developing rhabdomyolysis, although it is less common than in patients with malignant hyperthermia.7 Those individuals who were taking an antipsychotic medication as a cause for their NMS can be treated with bromocriptine, a dopamine agonist used to overcome dopaminergic blockade. There are case reports for the use of dantrolene in NMS. However, this remains controversial. Parkinson’s disease patients who present with NMS secondary to withdrawal should be restarted on their appropriate dopamine agonist medications. In addition, aggressive IV fluid hydration can help prevent acute renal failure and enhance excretion of muscle breakdown products. Each year, approximately 2,000 patients are diagnosed with NMS in the United States, with an overall mortality rate of approximately 10 percent.8 Disposition of patients with NMS, as with the other hyperthermic disorders, involves close monitoring in an intensive care unit.
Serotonin Syndrome
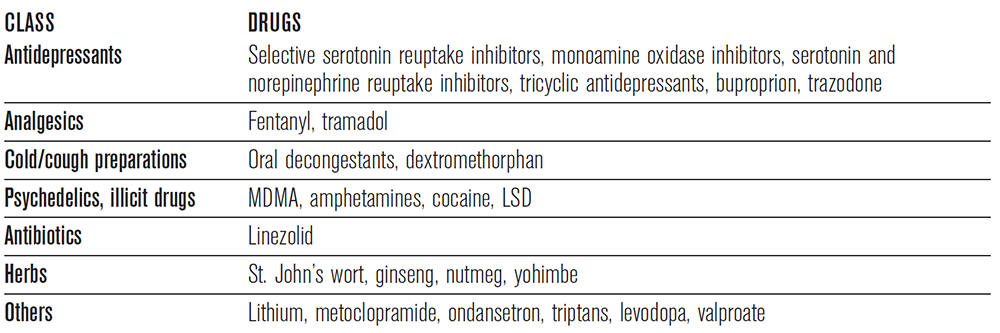
Case 3 represents a patient with serotonin syndrome. Serotonin syndrome presents as a constellation of cognitive, neuromuscular, and autonomic symptoms secondary to an excess of synaptic serotonin. The mechanism of this syndrome is quite complex and involves a combination of interactions involving central catecholamine release, the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, and skeletal muscle. Often, as described in this case, the syndrome is seen in patients who ingest medications that synergistically increase serotonin, although it can also occur from an overdose of a single serotonergic agent.9 Some of these drugs are listed in Table 2.
The symptoms of serotonin syndrome are extremely variable, ranging from mild presentations to a life-threatening syndrome. The most common presenting symptoms are muscle rigidity, altered mental status, and hyperthermia. Classically, and differentiating this condition from the other hyperthermic disorders, patients develop myoclonus, which is characteristically more evident in the lower extremities. The onset of symptoms is typically rapid, with symptoms typically presenting within minutes to hours. The cornerstone to management of serotonin syndrome involves immediate discontinuation of all serotonergic agents as well as supportive treatment with aggressive cooling and benzodiazepines. Current recommendations include the use of cyproheptadine, an antihistamine with antiserotonergic properties, and chlorpromazine, a phenothiazine antipsychotic with antiserotonergic properties.10 Mortality rates are similar to that of NMS, between 2 and 12 percent.
Treatment
Management of patients with the described toxin-induced hyperthermic disorders begins with supportive care and focuses on decreasing muscle activity (benzos) and core body temperature (and chill). Benzodiazepines play an important role in decreasing mortality by reducing shivering and muscle breakdown that can lead to rhabdomyolysis, hyperkalemia, and ultimately renal failure. Patients with severe toxicity who do not respond to benzodiazepines may even require chemical paralysis with a non-depolarizing paralytic agent along with mechanical ventilation for better control of their muscle hyperactivity.
Dr. Traficante is a PGY-3 resident and Dr. Kashani is a physician in the department of emergency medicine at St. Joseph’s Regional Medical Center in Paterson, New Jersey.
References
- MacLennan DH. The genetic basis of malignant hyperthermia. Trends Pharmacol Sci. 1992;13(8):330-334.
- Rosenberg H, Davis M, James D, et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21.
- Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110(2):498-507.
- Britt BA, Kalow W. Malignant hyperthermia: a statistical review. Can Anaesth Soc J. 1970;17:293-315.
- Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228.
- Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156(2):169-180.
- Modi S, Dharaiya D, Schultz L, et al. Neuroleptic malignant syndrome: complications, outcomes, and mortality. Neurocrit Care. 2016;24(1):97-103.
- Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120. Erratum in: N Engl J Med. 2007;356:2437.
Pages: 1 2 3 4 | Multi-Page



No Responses to “Tips for Diagnosing,Treating Toxin-Induced Hyperthermic Disorders”