ACEP’s “Clinical Policy: Use of Intravenous Tissue Plasminogen Activator for the Management of Acute Ischemic Stroke in the Emergency Department” was approved by the Board of Directors in its June 24, 2015, meeting in Dallas. The 2015 tissue plasminogen activator (tPA) policy revised one passed in 2012.
Explore This Issue
ACEP Now: Vol 34 – No 08 – August 2015A writing subcommittee of the ACEP Clinical Policies Committee, chaired by Michael D. Brown, MD, FACEP, conducted a systematic review of the literature to derive evidence-based recommendations to answer two clinical questions. The table (right) displays the 2015 draft recommendations on the left and the final published recommendations, which incorporate feedback from the ACEP member comment period, on the right.
Among the 50 comments received on the draft policy, some were more impactful than others. For example, some appropriately pointed out that the draft Level A harm statement was not action oriented, which is one of the primary aims in developing recommendations that are useful to clinicians.
—Michael D. Brown, MD, FACEP
As Dr. Brown explained, “Among the 50 comments received on the draft policy, some were more impactful than others. For example, some appropriately pointed out that the draft Level A harm statement was not action oriented, which is one of the primary aims in developing recommendations that are useful to clinicians. Therefore, the statement regarding considering the risk of sICH associated with tPA was moved to follow the action-oriented Level B recommendations. Other comments highlighted the fact that the risk of sICH is dependent upon the characteristics of the individual patient; thus, including average estimates of risks in the recommendation could be misleading. However, estimates for potential benefit and harm (ie, number needed to treat, number needed to harm) based on the clinical trial with the highest class of evidence for each critical question (Class I for Question 1 and Class II for Question 2) were retained in Appendix D as examples of how one might initiate the process of shared decision making.”
So what in the original 2012 policy was changed in the 2015 policy?
Here’s a summary of the updates:
- This revised policy utilized an updated clinical policy development process that includes formally trained methodologists grading the research evidence.
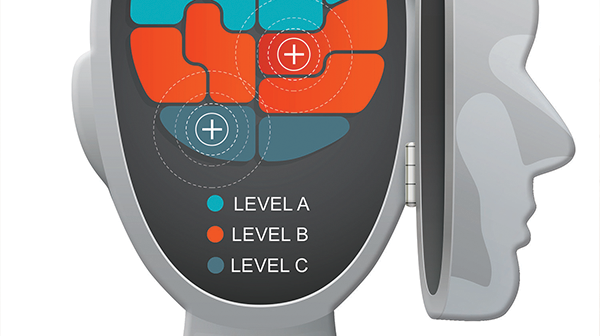
- There were no Level A recommendations since this level requires a high degree of clinical certainty.
- Although Level B recommendations reflect a moderate degree of clinical certainty, the purposeful choice of wording in the recommendations differentiates the action-oriented statements for Question 1 and Question 2.
- The Level C recommendations, based on consensus opinion, emphasize the importance of shared decision making when feasible.
- The potential for benefit and harm associated with the use of intravenous tissue plasminogen activator in acute ischemic stroke is summarized in Appendix D of the policy.
This clinical policy is posted on the ACEP Web site at www.acep.org/clinicalpolicies. It will be published in Annals of Emergency Medicine in September 2015.
Pages: 1 2 | Single Page





One Response to “ACEP Board Approves tPA Clinical Policy”
April 10, 2018
Podcast #90 - Indefinite Evidence and Biased Reporting « FOAM EMS[…] The American College of Emergency Physicians in 2015 did update their guidelines and did so where “There were no relevant industry relationships disclosed by the subcommittee members” which is very different than most other groups. They were not able to make any Level A recommendations but did make Level B and C recommendations. This matters for a number of reasons but ultimately they talked about how tPA “should be offered and may be given” to select patients within three (3) hours of symptom onset and that “tPA may be offered and may be given to carefully selected patients” within 3 to 4.5 hours after symptom onset in appropriate centers. In both cases, shared decision making is encouraged when feasible. The reason why this matters is that the liberal language allows for more careful consideration based on the complex nature of this disease process to help tailor care to individual patients. […]