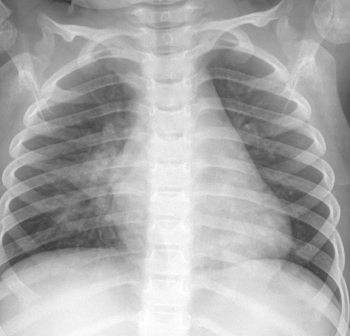
Question: Do steroids improve clinical outcomes in cases of pediatric pneumonia?
A Cochrane meta-analysis by Stern et al suggests that corticosteroids in adults with severe community-acquired pneumonia (CAP) appear to significantly reduce mortality.1 Is this true in children?
Explore This Issue
ACEP Now: Vol 38 – No 04 – April 2019A 36-hospital multicenter study by Weiss et al retrospectively evaluated 20,703 children ages 1 to 18 years.2 The primary outcomes were length of stay (LOS), readmission within 28 days, and total hospital cost in patients who received antibiotics with and without systemic corticosteroids. Overall, systemic corticosteroid therapy was associated with a decrease in LOS (hazard ratio [HR] 1.26; 95% CI, 1.20–1.32). Beta agonists alone were not associated with a significant difference in LOS, but in patients who received beta agonists at initial presentation, the addition of systemic corticosteroids did decrease LOS (HR 1.36; 95% CI, 1.28–1.45). In children who did not receive beta agonists, adjunct systemic corticosteroids were associated with a significantly longer LOS (HR 0.85; 95% CI, 0.75–0.96). These findings may suggest a concomitant reactive airway disease or asthma component affecting LOS in these children with CAP.
An outpatient clinic study by Ambroggio et al studied adjunct systemic corticosteroids with antibiotics in CAP.3 The authors retrospectively studied 2,244 children, and treatment failure leading to hospital admission occurred in only two patients.
A multicenter, randomized, double-blind, placebo-controlled trial by Tagarro et al evaluated the effects of dexamethasone (0.25 mg/kg/dose every six hours for 48 hours) on CAP with parapneumonic pleural effusions in children ages 1 month to 14 years (n=57).4 The primary endpoint was time to recovery (in hours), defined as oxygen saturations greater than 92 percent, continuous temperature less than 37ºC, no respiratory distress, end of invasive procedures, pneumonia in resolution, and oral feeding. Secondary outcomes included complications or changes in pleural effusion size. The median time to recovery in patients receiving dexamethasone versus placebo was 109 versus 177 hours (P=0.037), respectively, 68 hours (3.1 days) shorter in the dexamethasone group. This benefit was predominantly found within the “simple effusion” group compared with the “complex effusion” group. Complex parapneumonic effusions were defined as pH less than 7.2, loculations or septations on ultrasounds, or bacteria on gram stain of pleural fluid. There were no significant differences in adverse events with the exception of hyperglycemia requiring insulin in one child in the dexamethasone group. This study suggests that the addition of dexamethasone shortens recovery time in children with parapneumonic pleural effusions.
Finally, the meta-analysis by Stern et al mentioned earlier was not able to make any conclusions about pediatric mortality; no children died in any of the three studies pooled.1 Regarding time to clinical cure in children with bacterial pneumonia, the pooled results suggest benefit from corticosteroids (–1.57 days; 95% CI, –2.55 to –0.60). Early clinical failure in children is also favored by the addition of corticosteroids (risk ratio 0.41; 95% CI, 0.24–0.70). It is important to note that one of the studies included in the pooled results of this meta-analysis focused on refractory Mycoplasma pneumoniae pneumonia, defined as clinical failure after being on macrolide therapy for seven days prior to inclusion.5 This study accounts for approximately 38 percent of the pooled analysis and may not apply to our patient population.
Pages: 1 2 | Single Page



One Response to “Corticosteroids for Pediatric Pneumonia”
October 22, 2019
Matt JaegerBut the real question is whether or not a “perihilar infiltrate” in a child represents a treatable infection at all. It certainly is not highly suggestive of a bacterial infection and based on the patient’s clinical appearance I would suggest that the best course for most children with an X-ray of this appearance is supportive care only.