Whether in the context of febrile illness, mild delirium, or the dreaded “weak and dizzy,” sepsis lurks around every corner. Then, in an era replete with serious respiratory viruses such as SARS-CoV-2, influenza, and respiratory syncytial virus, the challenge persists of differentiating systemic viral illness from bacteremia. However, where practicing clinicians see problems, diagnostics companies see opportunities.
Explore This Issue
ACEP Now: Vol 41 – No 07 – July 2022Diagnostic and Supplemental Testing
Most emergency physicians are well acquainted with the process of teasing out a diagnosis of infection from otherwise deranged physiology, and likewise further clarifying an underlying bacterial source. As the pressures mount for ever-earlier intervention and even greater diagnostic accuracy, clinical evaluation is supplemented by laboratory testing. Generationally, the simplest tool remains the complete blood count (CBC) and differential, using the white blood cell count and its differentiation between neutrophils, lymphocytes, and other immature forms as clues to further inform the presence and type of infection.
The well-described limitations in sensitivity and specificity for the CBC have led further afield to supplementary tests. Most commonly, and dependent upon local practice patterns, these supplemental tests are typically C-reactive protein (CRP) and procalcitonin. These non-specific markers of systemic inflammation provide incremental predictive value in determining the presence of a serious bacterial infection. Unfortunately, each of these tests generally displays a normal result in concert with a correspondingly benign clinical picture, and a grossly abnormal result when infection is clearly present. In cases where a diagnosis is less clear, results from these tests tend to land squarely in uninformative, indeterminate ranges. Furthermore, each test may be confounded by chronic inflammatory conditions, or falsely reassuring in immunosuppressed patients and early in a disease process.
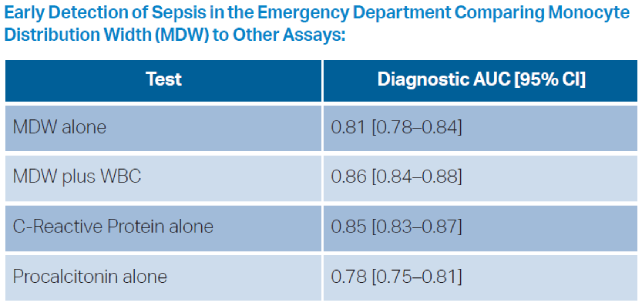
Despite the marketing push behind procalcitonin over the past decade, growing recognition of its limitations has led to the development of several novel objective tools attempting to improve upon the current state of disarray. One of these is the monocyte distribution width (MDW), branded by Beckman Coulter as the Early Sepsis Indicator (ESId).1 Similar to technologies in automated analyzers in which leukocyte type and red cell size can be evaluated, MDW can likewise be observed. Because monocytes with inflammatory phenotypes increase in size, and these changes may be observed in response to sepsis, MDW has been proposed as another early marker of sepsis.
This novel measurement has ultimately shown little added value over current nonspecific markers. Across various studies evaluating its performance, the area under the receiver operating curve (AUROC) for MDW is in the range of 0.70 to 0.80.2 While this has better diagnostic precision than a coin flip, in various retrospective and prospective evaluations MDW performed similarly to both CRP and procalcitonin.3 At the MDW cut-off value of 20 (defined at regulatory approval), sensitivity is reported as 95.5 percent, with a specificity of 26.5 percent. This product thus slots in precariously as a one-way decision tool to reinforce a clinical decision of the absence of sepsis, but with extremely poor positive predictive value.4 The primary advantage of this test compared to CRP or procalcitonin, is that the result is embedded in the CBC, rather than necessitating a separate assay.
Pages: 1 2 3 4 | Single Page






No Responses to “Diagnosing Sepsis, the Next Generation”