Generally speaking, all of these potential tools fall far short of demonstrating their value. Each shows some predictive power to address their problem of interest. Many lack prospective, operational comparisons with presently available objective adjuncts to clinical judgment, such as procalcitonin and CRP, and none robustly demonstrate superiority. Most importantly, no studies of these tests report on prospective implementation and impact on either surrogates for sepsis management, such as antibiotic administration and appropriateness, or patient-oriented outcomes relating to morbidity or mortality. While these are exciting times to witness the development of new tools, remain cautious about adoption before their value is proven.
Explore This Issue
ACEP Now: Vol 41 – No 07 – July 2022Dr. Radecki is an emergency physician and informatician with Christchurch Hospital in Christchurch, New Zealand. He is the Annals of Emergency Medicine podcast co-host and Journal Club editor and can be found on Twitter @emlitofnote.
References
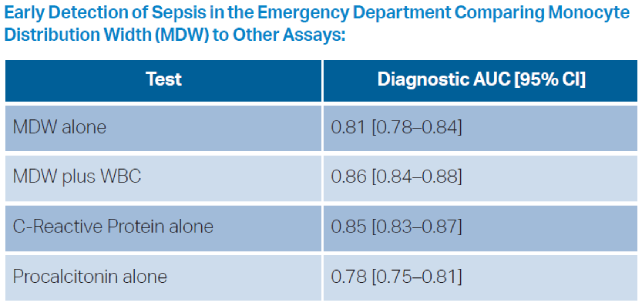
- Crouser ED, Parrillo JE, Seymour CW, et al. Monocyte distribution width: a novel indicator of sepsis-2 and sepsis-3 in high-risk emergency department patients. Crit Care Med. 2019;47(8):1018–1025.
- Piva E, Zuin J, Pelloso M, et al. Monocyte distribution width (Mdw) parameter as a sepsis indicator in intensive care units. Clin Chem Lab Med. 2021;59(7):1307–1314.
- Woo A la, Oh DK, Park CJ, Hong SB. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS One. 2021;16(4):e0250101.
- Crouser ED, Parrillo JE, Martin GS, et al. Monocyte distribution width enhances early sepsis detection in the emergency department beyond SIRS and qSOFA. J Intensive Care. 2020;8(1):33.
- Guillou L, Sheybani R, Jensen AE, et al. Development and validation of a cellular host response test as an early diagnostic for sepsis. PLoS One. 2021;16(4):e0246980.
- O’Neal HR, Sheybani R, Caffery TS, et al. Assessment of a cellular host response test as a sepsis diagnostic for those with suspected infection in the emergency department. Crit Care Explor. 2021;3(6):e0460.
- O’Neal HR, Sheybani R, Caffery TS, et al. Assessment of a cellular host response test to risk-stratify suspected COVID-19 patients in the Emergency Department setting. PLoS One. 2022;17(3):e0264220.
- Hassan U, Ghonge T, Reddy B, et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat Commun. 2017;8(1):15949.
- Taneja I, Reddy B, Damhorst G, et al. Combining biomarkers with emr data to identify patients in different phases of sepsis. Sci Rep. 2017;7(1):10800.
- Taneja I, Damhorst GL, Lopez-Espina C, et al. Diagnostic and prognostic capabilities of a biomarker and EMR-based machine learning algorithm for sepsis. Clin Transl Sci. 2021;14(4):1578–1589.
- van der Does Y, Rood PPM, Ramakers C, et al. Identifying patients with bacterial infections using a combination of C-reactive protein, procalcitonin, TRAIL, and IP-10 in the emergency department: a prospective observational cohort study. Clinical Microbiology and Infection. 2018;24(12):1297–1304.
- 510(k) Substantial Equivalence Determination Decision Summary [regulatory government]. United States Food and Drug Administration. 1 Sept 2021. Available at: https://www.accessdata.fda.gov/cdrh_docs/reviews/K210254.pdf. Accessed June 13, 2022.
Pages: 1 2 3 4 | Single Page





No Responses to “Diagnosing Sepsis, the Next Generation”