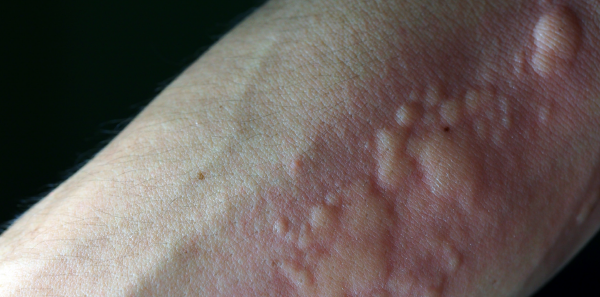
Should We Add It to Our Toolbox?
Having summarized the high-level observations from this trial, it is reasonable to conclude, as the FDA reviewers did, that IV cetirizine is safe and effective.2 The more challenging question addresses its appropriateness. The justification for its use in acute urticaria is stated by the authors as “the parenteral route of administration is often preferred,” citing a generalist American Family Physician review article that, interestingly enough, does not actually include such a statement.3 There is no evidence IV agents provide superior treatment of urticaria than oral ones for this frequent condition. In fact, the most recent practice update from the immunology community does not specify any specific route of administration for the most effective treatment of urticaria.4
Explore This Issue
ACEP Now: Vol 40 – No 06 – June 2021To this larger point, the original oral cetirizine pharmacology labeling information from Pfizer in 2002 described rapid onset of inhibition of histamine release reaching 95 percent effectiveness within one hour.5 Furthermore, the mean area under the concentration-time curve for the IV formulation is identical to that of the oral formulation. The obvious difference between the preparations is the IV form reaches a peak concentration of >300 percent the oral formulation and within minutes rather than an hour. The FDA reviewers, rather than commenting on a potential clinical benefit, focused instead on the safety profile of such a high serum level.
The bottom-line question: Does this product have a purpose? For its approved indication of acute urticaria, the most likely answer is almost never. Absent evidence to demonstrate the advantages of IV therapy over oral, the question asked by this trial is virtually moot, other than perhaps in patients unable to tolerate oral intake. For most patients, any rescue medications, such as H2 blockers or steroids, can just as easily be given by mouth. But what about the “time in treatment center”? As noted above, measuring this outcome obscures the practicalities of a busy emergency department, particularly the resources required for IV access in the first place. A patient presenting with acute urticaria can be treated with an oral second-generation antihistamine at triage and easily reach therapeutic serum levels in less time than an intravenous version could be administered. In other words, if the study measured time from triage, most of the outcomes would likely have been indistinguishable. The authors seem to have forgotten that avoiding an IV is itself a positive outcome.
But what about anaphylaxis? In these cases, IV access is usually obtained early anyway. But the consensus from the allergy and immunology community is quite clear (though markedly different from standards of care in most EDs) that neither antihistamines nor steroids have any important role in treatment of anaphylaxis and certainly need not be given intravenously. A patient with an uncomfortable urticarial component to anaphylaxis not resolving with epinephrine is just as well-served by oral antihistamines. And those patients are not being discharged in an hour.
The final tiebreaker, of course, is always cost. The wholesale acquisition cost of Quzyttir is reportedly $300 for a 10-mg vial, which, combined with additional nursing resource charges for intravenous access and medication administration, rapidly increases the total cost for an acute encounter.6 Conversely, if prudent avoidance of therapies lacking clear added value is important, then continued routine use of generic oral second-generation antihistamines will serve just as well.
The opinions expressed herein are solely those of Dr. Radecki and do not necessarily reflect those of his employer or academic affiliates.
Pages: 1 2 3 | Single Page




2 Responses to “Does New IV Urticaria Medication Offer Benefits Over Current Treatments?”
July 19, 2021
Dr. Joseph SachterExcellent review — as usual. Although I might use the intravenous preparation a little more frequently than “almost never’ (an unaccompanied patient who drove to themselves to the ED and might otherwise be ready for discharge a couple of hours after treatment initiation), the $300 a dose price is a dealbreaker.
This in turn brings up an interesting economic question. Wouldn’t TerSera (marketers of the drug) make more money overall if they charged less? Best analogy I can think of is a movie theatre charging $5 for a pint of spring water that costs them no more than a dime. Wouldn’t they sell at least five times more if they charged a dollar?
July 20, 2021
Ryan RadeckiI would imagine the price point for Quzyttir was thoughtfully selected from a team of analysts putting together a value-based price – potentially incorporating, perhaps, the revenue gain from reduced ED LOS if the drug were to offer some more rapid discharge, etc.