History of Present Illness
A 67-year-old female presented to a community emergency department with headache and left-sided ptosis. Her headache started two weeks ago and was gradual in onset. She described it as her typical migraine: a sharp pain that was worse with light and associated with nausea and vomiting. She used sumatriptan, which improved her symptoms after several days. Three days prior to her presentation to the emergency department, she developed recurrence of this headache with left-sided ptosis and left-sided facial numbness. The patient denied fever, chills, vision changes, neck stiffness, chest pain, abdominal pain, syncope, slurred speech, difficulty ambulating, trauma and recent illness. Her past medical history was significant for invasive ductal carcinoma of the breast (status post lumpectomy and radiation therapy, in remission), migraines, benign paroxysmal positional vertigo and hypertension.
Explore This Issue
ACEP Now: Vol 41 – No 05 – May 2022A computed tomography angiography (CTA) of the head and neck was obtained to rule out a cerebrovascular accident. This imaging revealed two intracranial aneurysms, with the first involving the posterior communicating artery (PCOM) and the second involving the internal carotid artery (ICA). It also revealed severe stenosis of the M2 branch of the right middle cerebral artery (MCA). Concerned for subarachnoid hemorrhage (SAH), the physician transferred the patient to our emergency department for further management.
Physical Exam
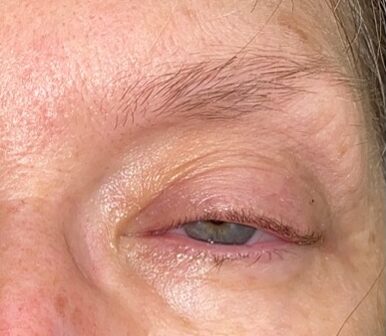
Vitals signs demonstrated no fever, blood pressure 170/103, tachycardia of 110 and oxygen saturation of 95–100 percent on room air. The patient was alert, oriented and in no acute distress. Her airway was intact with no respiratory distress. Equal pulses were appreciated in all extremities. Her neurological exam was notable only for left-sided ptosis and decreased sensation to light touch to the left side of her forehead, cheek and chin. There were no other cranial nerve deficit, no focal weakness about her extremities and no ataxia. No meningismus was appreciated. The left eye demonstrated ptosis without periorbital edema, erythema, proptosis, chemosis, conjunctival injection or discharge (Figure 1). Cardiac, respiratory and abdominal exams showed no acute pathological finding. Additionally, there were no external findings of infection or trauma.
Workup and Hospital Course
Labs were obtained and included a complete blood count with differential, basic metabolic panel, coagulation panel and magnesium. No acute abnormalities were identified. Neurosurgery was consulted. They initially recommended an MRI of the brain with and without contrast. This demonstrated the same PCOM and ICA aneurysms seen on the prior CTA, with no signs of intracranial hemorrhage. Despite symptom management with prochlorperazine and intravenous fluids, the patient’s headache persisted. With persistent concern for a SAH, despite an MRI negative for acute intracranial bleeding, a lumbar puncture was performed. This was positive for xanthochromia. Tube one had a red blood cell count (RBC) of 5,270, and tube two had a RBC of 4,426. This confirmed the diagnosis of SAH, likely secondary to a ruptured aneurysm. Neurosurgery recommended admission to the operating suite for digital subtraction angiography and endovascular coiling. In the emergency department, the patient’s systolic blood pressure was kept below 140 (using push doses of labetalol), levetiracetam was administered for seizure prophylaxis and nimodipine was given in an attempt to decrease chances of delayed ischemia.
In the operating suite with interventional radiology, the patient was noted to have a single bilobed aneurysm of her PCOM (Figure 2). There was no separate ICA aneurysm, as previously reported on CTA and MRI. The aneurysm was secured via coiling with no complications. The patient was transferred to the intensive care unit for close monitoring. Her ptosis gradually improved with no new deficits.
The patient was discharged home seven days post-op with improved but persistent ptosis. Follow-up appointments were scheduled with neurosurgery, neurology and her primary care physician. Two weeks following discharge, at her neurology appointment, the patient was found to have complete resolution of ptosis and her facial sensory deficit.
Discussion
SAH is a devastating neurological emergency defined by extravasation of blood into the subarachnoid space. It can be divided into two categories: aneurysmal and nonaneurysmal. As our case involved aneurysmal SAH, the remainder of this discussion will focus on the diagnosis and management of this category of SAH.
With SAH mortality estimated from 21.5 percent to 37.8 percent, correct diagnosis is crucial in patients presenting with a nontraumatic headache.1, 2 The Ottawa SAH rules have been proposed to help rule out SAH in this group of patients. If all criteria are met, this set of rules was found to be 100 percent sensitive to rule out SAH.3 However, as there has yet to be external validation of these rules, there is concern with using this clinical tool to consistently rule out SAH.
If there is suspicion for SAH, the initial diagnostic study of choice is a noncontrast CT of the head. If obtained within six hours of symptom onset, this imaging modality is 98.7 percent to 100 percent sensitive in ruling out SAH.4, 5 When CT is obtained 12 to 24 hours from symptom onset, the sensitivity decreases to 81.7 percent to 93 percent.6,7
There is ongoing debate within emergency medicine regarding subsequent diagnostic approaches if head CT is negative for SAH. Lumbar puncture has been the traditional next step. Classic findings are an elevated opening pressure, xanthochromia and RBCs that remain elevated from tube one to tube four. Xanthochromia can be determined by visual inspection or spectrophotometry and may not be appreciated until several hours after hemorrhage. The presence of xanthochromia with greater than 2,000 RBCs has 100 percent sensitivity for SAH.8 Additionally, if the RBC count in tube four is 47 percent or higher than that in tube one, this is highly suggestive of SAH.9 If the count is 30 percent or lower, this reliably excludes SAH and favors a traumatic lumbar puncture.8
There is growing support for using head and neck CTA alone to rule out SAH. Generally, a concerning history and physical combined with identification of an aneurysm on CTA is highly suggestive of SAH. This may be an acceptable alternative to lumbar puncture in the diagnosis of SAH.10 The concern with this approach is the possible unnecessary intervention on asymptomatic aneurysms. The official policy from ACEP recommends either lumbar puncture or CTA to safely rule out SAH after shared decision making with the patient regarding the risks and benefits of each modality.11
Management of aneurysmal SAH in the emergency department involves stabilizing the patient until definitive treatment with coiling or clipping can be performed. The mainstay of treatment is blood pressure control and reversal of anticoagulation. A goal systolic blood pressure (SBP) less than 160, or mean arterial pressure (MAP) less than 110, is currently recommended.12 Although some studies have shown improved outcomes with a goal SBP less than 140, recent data have suggested a higher risk of rebleeding when compared to a SBP goal of less than 160.13,14 Avoiding hypotension is equally important. A low MAP combined with increased intracranial pressure can severely decrease the cerebral perfusion pressure.
Cessation of anticoagulation with appropriate reversal should be initiated based on the patient’s active medication regimen.15 All therapies should be considered in the context of timing and size of last dose and should be given in coordination with neurology or neurosurgery. Warfarin is reversed with intravenous vitamin K and prothrombin complex concentrate (PCC). If antiplatelet therapy is used, or the platelet count is less than 100,000/µL, one apheresis unit of platelets should be administered. Desmopressin is also used for antiplatelet reversal based on observed benefits in several small studies, its low cost and favorable side effect profile. However, data are limited. Factor Xa inhibitors can be reversed with andexanet alfa. This therapy was still in development at the time of publication of the Neurocritical Care Society’s guidelines. Thus, PCC is still recommended for Factor Xa reversal with consideration of active charcoal administration (if the patient is intubated). Direct thrombin inhibitors are typically reversed with PCC as well, with consideration of charcoal (if the patient is intubated). Special consideration exists for dabigatran, which can be reversed with idarucizumab.15
Other facets of management are more controversial and should be made in conjunction with neurology or neurosurgery. Seizure prophylaxis is often considered, especially in the immediate posthemorrhage window.12 Nimodipine is indicated to avoid delayed cerebral ischemia. However, this can be safely initiated within four days of hemorrhage onset and is therefore not a crucial component of ED care.16 Strict glycemic control, avoiding hypo- and hypernatremia, steroid use and magnesium sulfate use have varied recommendations and are often beyond the scope of acute ED management of SAH.
References
- Korja M, Silventoinen K, Laatikainen T, et al. Cause-specific mortality of 1-year survivors of subarachnoid hemorrhage. Neurology. 2013;80(5):481-486.
- Chan V, Lindsay P, McQuiggan J, et al. Declining admission and mortality rates for subarachnoid hemorrhage in Canada between 2004 and 2015. Stroke. 2019;50(1):181-184.
- Perry JJ, Stiell IG, Sivilotti MLA, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA. 2013;310(12):1248-1255.
- Dubosh NM, Bellolio MF, Rabinstein AA, et al. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke. 2016;47(3):750-755.
- Perry JJ, Stiell IG, Sivilotti MLA, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277.
- van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology. 1982;23(3):153-156.
- Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3(9):827-831.
- Perry JJ, Alyahya B, Sivilotti MLA, et al. Differentiation between traumatic tap and aneurysmal subarachnoid hemorrhage: prospective cohort study. BMJ. 2015;350:h568.
- Czuczman AD, Thomas LE, Boulanger AB, et al. Interpreting red blood cells in lumbar puncture: distinguishing true subarachnoid hemorrhage from traumatic tap. Acad Emerg Med. 2013;20(3):247-256.
- Meurer WJ, Walsh B, Vilke GM, et al. Clinical guidelines for the emergency department evaluation of subarachnoid hemorrhage. J Emerg Med. 2016;50(4):696-701.
- Godwin SA, Cherkas DS, Panagos PD, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2019;74(4):e41-e74.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711-1737.
- Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365.
- Oheda M, Inamasu J, Moriya S, et al. Early rebleeding in patients with subarachnoid haemorrhage under intensive blood pressure management. J Clin Neurosci. 2015;22(8):1338-1342.
- Frontera JA, Lewin JJ 3rd, Rabinstein AA, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24(1):6-46.
- Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20(1):277.
Dr. Effron is assistant professor of emergency medicine at Case Western Reserve University and attending physician in the department of emergency medicine at the MetroHealth Medical Center, in Cleveland, Ohio. DR. YAVORSKY is an emergency physician in Cleveland, Ohio.


No Responses to “Ptosis and Subarachnoid Hemorrhage: Opening an Eye to Intracranial Hemorrhage”