The Case
A 73-year-old man currently undergoing chemotherapy for colon cancer presents to the emergency department with a swollen right leg. His vital signs are all stable, and his leg does not look like it has cellulitis. The ultrasound confirms your suspicion of a deep-vein thrombosis (DVT). The patient is being prepared for outpatient management, but does not want to be on injections for the next six to 12 months. He asks if there are any other treatments besides low-molecular-weight heparin (LMWH).
Explore This Issue
ACEP Now: Vol 38 – No 01 – January 2019Background
Cancer increases the risk of venous thromboembolism (VTE). Patients with cancer can be difficult to manage due to the higher risk of bleeding and the higher rate of thrombosis recurrence. The CLOT trial established LMWH as the standard therapy for symptomatic and asymptomatic VTE.1
Direct oral anticoagulants (DOACs) like rivaroxaban have been shown to be effective treatments for VTE without causing increased bleeding complication rates in non-cancer patients. A trial by Bean et al suggested it was safe and effective to dry start DOACs (no LMWH needed) in certain patients with VTE.2
Although DOACs are frequently used in the treatment of cancer-associated VTE, there is little evidence to support this practice. The SELECT-D trial was a small open-label pilot trial looking at the use of rivaroxaban. It showed a lower hazard ratio (HR) for VTE with wide confidence intervals and a higher clinically relevant non-major bleeding rate.3
Clinical Question
In cancer-associated VTE, is edoxaban noninferior to LMWH?
Reference
Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624.
- Population: Adult patients with active cancer or cancer diagnosed within the previous two years with acute symptomatic or asymptomatic VTE.
- Exclusions: See the article’s supplementary appendix.
- Intervention: LMWH for five days followed by oral edoxaban 60 mg daily for at least six months.
- Comparison: Subcutaneous (SQ) dalteparin 200 IU/kg daily (maximum 18,000 IU) for one month followed by 150 IU/kg daily for at least five months.
- Outcome:
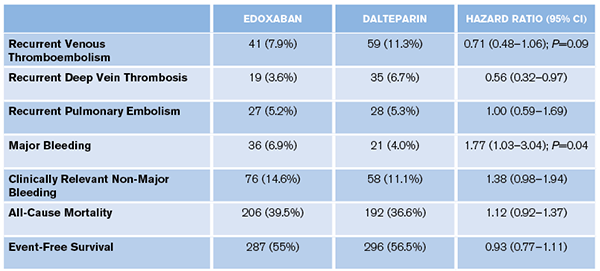
- Primary: Composite of recurrent VTE or major bleeding during 12-month follow-up.
- Secondary: Clinically relevant non-major bleeding (CRNB), event-free survival, VTE-related death, all-cause mortality, recurrent DVT, recurrent pulmonary embolism. (The complete list is in the article’s supplementary appendix.)
Authors’ Conclusions
“Oral edoxaban was noninferior to subcutaneous dalteparin with respect to the composite outcome of recurrent venous thromboembolism or major bleeding. The rate of recurrent venous thromboembolism was lower but the rate of major bleeding was higher with edoxaban than with dalteparin.”
Pages: 1 2 3 | Single Page




One Response to “Should You Use Direct Oral Anticoagulants for Cancer-Associated VTE?”
January 20, 2019
Gregg ChesneyThanks for this review! I had this exact debate last week (but enoxaparin vs rivaroxaban) with our ED pharmacist, the patient’s oncologist and his general surgeon for a patient with an acute DVT 2 weeks post-op from a resection of a colonic adenocarcinoma. I hadn’t seen the new article in the NEJM yet, and the 2016 ACCP VTE guidelines are still recommending LMWH. I had originally ordered enoxaparin but the oncologist decided he wanted to manage him on rivaroxaban, citing new data that DOACs are noninferior in these patients, which I hadn’t had a chance to look for yet, so thanks for filling me in!