
Explore This Issue
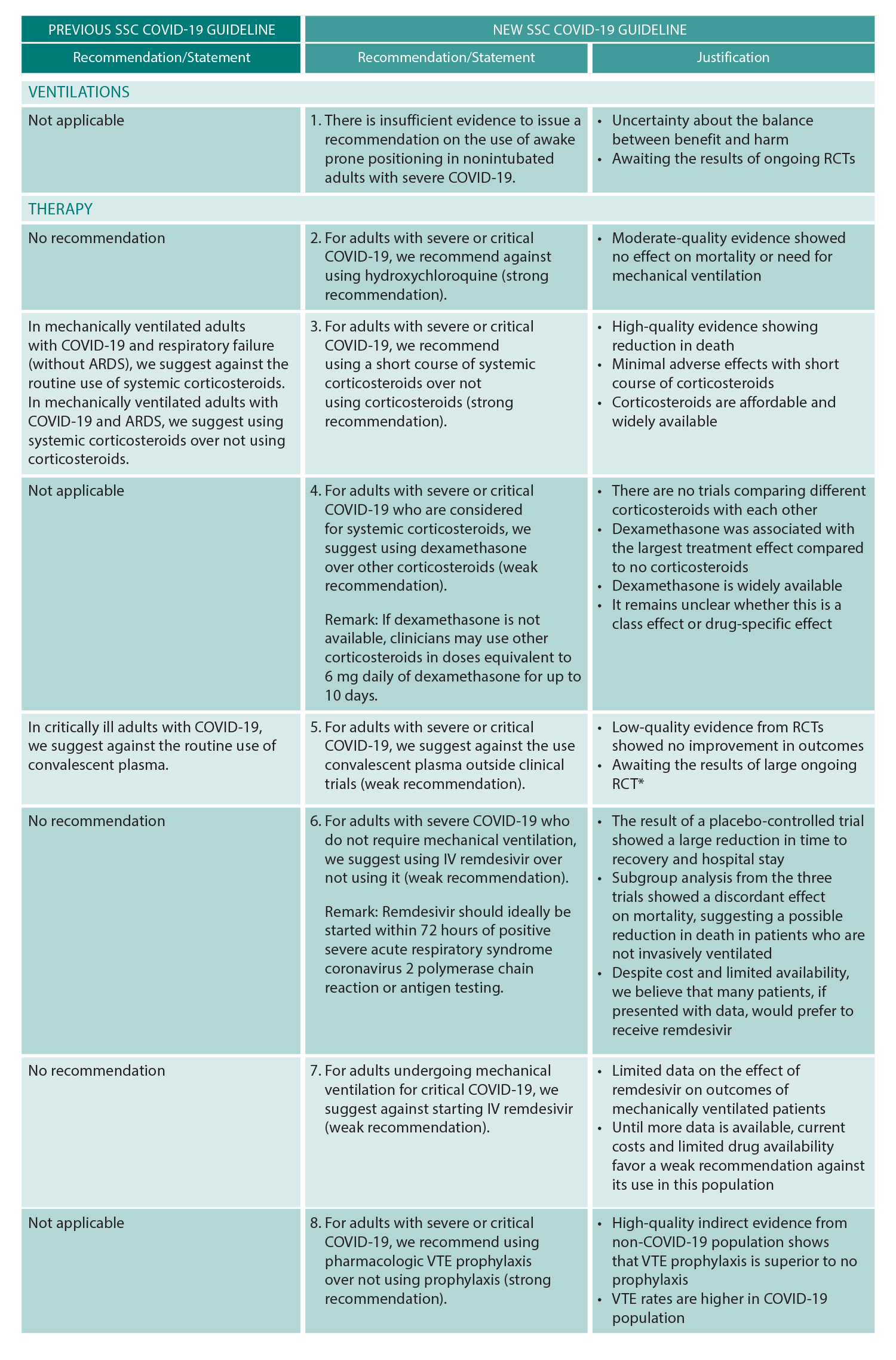
ACEP Now: Vol 40 – No 11 – November 2021(click for larger image) Table 1: SSC COVID-19 Guidelines
ARDS = acute respiratory distress syndrome, RCT = randomized controlled trial, VTE = venous thromboembolism.
Reprinted with permission from Crit Care Med. 2021;49(3):e219-e234.
* Editor’s note: The data regarding COVID changes rapidly. A large RCT, the C3PO trial, showed negative effects of convalescent plasma in August 2021.
The guideline utilizes “we recommend” for strong recommendations and “we suggest” for weak recommendations. Ultimately three new recommendations and six updated recommendations were added to the prior SSC COVID-19 guidelines. New and updated recommendations will be forthcoming as the COVID-19 evidence base grows in accordance with the above stated living guideline methodology.
Briefly, the guideline recommends against the use of hydroxychloroquine and therapeutic anticoagulation; suggests against convalescent plasma; recommends the use of pharmacologic venous thromboembolism prophylaxis and corticosteroids, suggesting dexamethasone as the corticosteroid choice; and suggests remdesivir in severe COVID-19 patients not needing mechanical ventilation. See Table 1 for a summary of the guideline updates.
The guideline uses the WHO definition of severe covid which, in adults, includes clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 <90 percent on room air.5 Plus-circle
Dr. Hickey is EMRA Representative to the Clinical Policies Committee 2019–2021. Dr. Villars is EMRA Representative to the Clinical Policies Committee 2021–2022.
References
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887.
- Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign: guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219-e234.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
- Moberg J, Oxman AD, Rosenbaum S, et al. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45.
- World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020 (No. WHO/2019-nCoV/clinical/2020.5). New York, NY: World Health Organization; 2020.
- Korley FK, Durkalski-Mauldin V, Yeatts SD, et al; SIREN-C3PO Investigators. Early convalescent plasma for high-risk outpatients with Covid-19 [online ahead of print Aug 18]. N Engl J Med. 2021.
Pages: 1 2 | Single Page



No Responses to “The Surviving Sepsis Campaign Creates COVID-19 Guideline”