For example, the concentration of older RIG formulations is 150 IU/mL. For a 75 kg person, 20 IU/kg requires 1,500 IU in 10 mL. Imagine trying to infiltrate a 10 mL syringe of RIG into the tip of a finger. Alternatively, the concentration of HyperRAB is 300 IU/mL, which in this example would equate to half of the previous amount, or 5 mL; it would still be difficult to get all of it in a finger wound, but twice as much could be injected. For my friend, this would represent the difference between having a small bump on his forehead or one that more resembles Pott’s puffy tumor. According to Red Book prices, the new HyperRAB formulation costs the same as the older HyperRAB S/D and the other RIG products, about $3,200 for 1,500 IU.
Explore This Issue
ACEP Now: Vol 37 – No 09 – September 2018If the price that hospitals and insurers negotiate is similar among formulations, pharmacies may decide to stock HyperRAB, both for its theoretical greater effectiveness by allowing more immunoglobulin to be injected into the wound and for the less pain associated with the remaining lower-volume intramuscular injection.
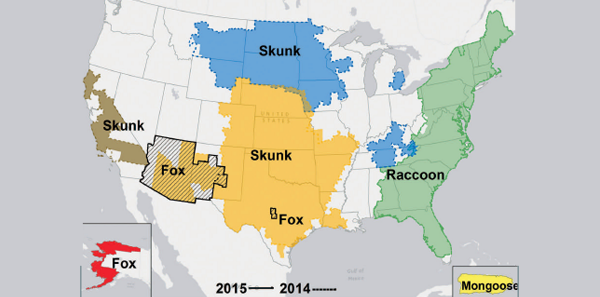
Rabies remains endemic in raccoons, foxes, and skunks regionally in the United States (see Figure 1) and bats everywhere, and each year between 60 and 70 dogs and more than 250 cats are found rabid. However, only 23 cases of human rabies have been reported in the past decade compared to 60,000 cases annually worldwide, and none were due to RPEP failure. So it does not appear that our previously available RIG preparations have been ineffective. RPEP failures have rarely occurred due to RIG injected only intramuscularly and not into all the wounds.
There are potential drawbacks with higher-concentrated RIG. For example, for extensive wounds, the Advisory Committee on Immunization Practices recommends diluting RIG to ensure sufficient volume to infiltrate all of the wounds. In this scenario, the doubly concentrated formulation might require additional dilution, which must be done with 5 percent dextrose in water rather than normal saline. Thus, the standard concentration might be preferred in this situation. Also, the change in concentration may increase the risk of miscalculating the dosage if clinicians mistakenly use the previous standard concentration.
In addition to RIG, rabies vaccine should be administered intramuscularly in the deltoid area, 1 mL initially (day 0) and then again on days 3, 7, and 14 (also day 28 if the patient is immunocompromised). Two licensed vaccines are currently available in the United States: human diploid cell vaccine (Imovax Rabies, Sanofi Pasteur) and purified chick embryo cell vaccine (RabAvert, Novartis Vaccine and Diagnostics).
Pages: 1 2 3 4 | Single Page


No Responses to “Tips for Treatment Following Possible Rabies Exposure”