The Case
A 68-year-old man presents with epistaxis. He has no history of coagulopathy but does have a history of hypertension. His vital signs are blood pressure 154/92, heart rate 70, and respiratory rate 14. He is taking an ACE-I and hydrochlorothiazide 25 mg but is not on any antiplatelet agents or anticoagulants.
Explore This Issue
ACEP Now: Vol 33 – No 10 – October 2014Question
Is topical tranexamic acid (TXA) better than nasal packing for anterior epistaxis?
Background
About 60 percent of the population will experience a nosebleed, with a bimodal distribution of <10 years old and >60 years old. The most common cause of epistaxis is an anterior bleed from the Kiesselbach’s plexus.
Routine coagulation studies only need to be performed on those patients already taking anticoagulants or a refractory pediatric hemorrhage requiring admission. Follow the American College of Chest Physicians guidelines for emergent anticoagulation reversal in the ED.1
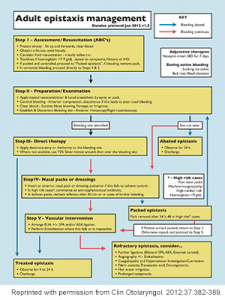
Antibiotics are not routinely recommended unless there is concurrent infection or posterior packing, and consider them for anterior packs left in place more than 24 hours. Refer to the Dundee protocol (see Figure 1) for general management of adult epistaxis.
Antibiotics are not routinely recommended unless there is concurrent infection or posterior packing,
Relevant Article
- Population: Adult ED patients
- Intervention: 15 cm cotton packing soaked in tranexamic acid (500 mg in 5 mL), removed after bleeding stopped
- Control: Cotton packing soaked in epinephrine (1:100,000) + lidocaine (2%) for 10 minutes and then repacked with cotton pledgets covered with tetracycline
- Outcome: Time to stop bleeding, length of stay (LOS), re-bleeding at 24 hours and one week, and patient satisfaction
- Authors’ Conclusion: “Topical application of injectable form of tranexamic acid was better than anterior nasal packing in the initial treatment of idiopathic anterior epistaxis.”
Quality Checklist for Randomized Control Trials
- The study population included or focused on those in the ED. Yes
- The patients were adequately randomized. Yes
- The randomization process was concealed. No
The authors write, “Because of the nature of the study, using different medications differing in consistency, color, and smell for soaking or coating the pledgets and discrepancy in the number of pledgets used, our physicians and patients were not blinded truly.” - The patients were analyzed in the groups to which they were randomized. Yes
- The study patients were recruited consecutively (ie, no selection bias). Yes
- The patients in both groups were similar with respect to prognostic factors. Unsure. More patients in the TXA group had a history of epistaxis (58 percent versus 14 percent).
- All participants (patients, clinicians, outcome assessors) were unaware of group allocation. No
- All groups were treated equally except for the intervention. Yes
- Follow-up was complete (ie, at least 80 percent for both groups). Yes
- All patient-important outcomes were considered. Yes
- The treatment effect was large enough and precise enough to be clinically significant. Yes
Comments
This was a good randomized controlled trial (RCT) looking at another way to treat a common problem in the ED. There was one significant imbalance in the two groups, with more patients in the TXA group having a history of epistaxis (58 percent versus 14 percent). This could exaggerate the effectiveness of the intervention.
Pages: 1 2 3 | Single Page





No Responses to “Is Topical Tranexamic Acid Better Than Nasal Packing for Anterior Epistaxis?”