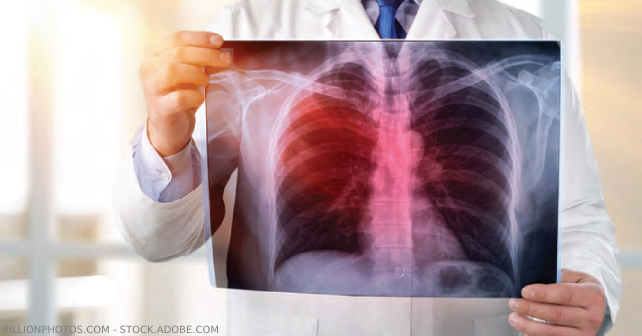
Although the treatment of community acquired pneumonia (CAP) is bread-and-butter emergency medicine, several guidelines and landmark studies have called for fairly big changes in clinical practice.1–4 Two recommendations deserve particular attention. Importantly, recommendations include significantly narrowing the use of antibiotics that cover methicillin resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (P. aeruginosa), even in severe pneumonia. That’s right, empiric vancomycin and piperacillin-tazobactam or cefepime only applies to a subset of “high risk” patients or those with severe pneumonia. Additionally, select patients with CAP requiring ventilatory support may benefit from hydrocortisone.
Explore This Issue
ACEP Now: Vol 42 – No 06 – June 2023Antibiotics
For years, antibiotic selection has been largely dependent on the presumed location or source of the development of the pneumonia. Categories of pneumonia have included CAP, hospital-acquired (HAP), ventilator-associated (VAP), and, beginning in 2005, health care-acquired (HCAP). Although these categories were created to help predict risk for multi-drug resistance (MDR), data demonstrated that patients who fell into the HCAP group did not have higher prevalence of antibiotic-resistant pathogens. As a result, the 2019 guidelines from the Infectious Disease Society of America (IDSA) significantly rein in those patients who should receive empiric coverage for MRSA or P. aeruginosa. The recommendations can be seen across categories, but notably empiric treatment with the beloved combination of vancomycin and piperacillin-tazobactam (or cefepime) is not recommended in CAP, even severe CAP, unless very specific risk factors are present.
For example, inpatients with a history of prior respiratory isolation of MRSA or P. aeruginosa should receive the addition of antimicrobial coverage targeted to the prior isolate, but not necessarily targeted to both MRSA and P. aeruginosa. Additionally, those with a hospitalization and receipt of parenteral antibiotics in the last 90 days should receive extended coverage for MRSA and/or P. aeruginosa only if they have specific locally identified risk factors for those organisms.
Lastly, given widespread macrolide resistance in the U.S., it’s unlikely that many clinicians are treating CAP with macrolide monotherapy. However, these guidelines also serve as a reminder that if you are routinely treating CAP with macrolide monotherapy and you are not certain that local resistance levels are less than 25 percent, it is time to choose a different antibiotic.
Corticosteroid Use
Historically, the administration of corticosteroids in patients hospitalized with pneumonia has been controversial. In 2017, the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) stated, “We suggest the use of corticosteroids for 5–7 days at a daily dose <400 mg intravenous hydrocortisone or equivalent in hospitalized patients with CAP,” citing moderate-quality evidence.2
Interestingly, the 2019 IDSA guidelines recommended that adults with CAP, including those with severe pneumonia, not routinely receive corticosteroids in the absence of refractory septic shock.1 What do we do with conflicting recommendations? Many clinicians, including myself, opted to wait for additional data–which we now have.
The tides have shifted towards administration of hydrocortisone to a select group of patients admitted with pneumonia. The CAPE COD trial, published early this year, demonstrated a 5.6 percent (95 percent CI, 1.7 to 9.6 percent) absolute reduction in mortality by day 28 in patients with severe CAP randomized to receive adjunct hydrocortisone 200 mg intravenously daily compared with those who received placebo.3 Additionally, fewer patients in the hydrocortisone arm were intubated, at 19.5 percent, compared with those in the placebo group, at 27.7 percent (Hazard Ratio, 0.69 [95 percent CI 0.60-0.94]). Although patients in the hydrocortisone group had more hyperglycemia, this did not translate into an increase in measurable adverse events. Crucially, to be included in the pivotal CAPE COD study, patients had to meet one of the following criteria: Pulmonary Severity Index score of >130, or ventilatory support (invasive mechanical ventilation, non-invasive ventilation (NIV), high-flow nasal cannula with fraction of inspired oxygen (FiO2) >0.5 and PaO2/FiO2 (P/F) ratio <300, or nonrebreather mask with a P/F ratio dependent on the oxygen flow rate).
The impressive results of the CAPE COD study should not be interpreted as “all hospitalized patients with CAP should receive steroids.” First, patient selection is critical. The patients in the CAPE COD trial were sick; nearly half of the cohort was intubated or on NIV. This is consistent with a 2018 meta-analysis of 11 trials that found steroids were associated with a reduced odds of death compared to control groups (relative risk, 0.66; 95 percent CI, 0.47-0.92). In subgroup analyses of severity of illness, the mortality benefit was only statistically significant in those with severe pneumonia. Further, the trial excluded those with influenza or aspiration pneumonia. Second, steroid choice is important. The CAPE COD trial used hydrocortisone, which has more mineralocorticoid activity than most other glucocorticoids. This may explain some differences between the results of CAPE COD and negative trials that used steroids with less mineralocorticoid activity.5 Third, hydrocortisone was early in the hospital course–an average of 20 hours from hospital admission. Future trials will provide additional insight, but in the interim, the data suggest we should administer hydrocortisone administration to patients who would meet the CAPE COD inclusion criteria.
 Dr. Westafer (@Lwestafer) is an attending physician and research fellow at Baystate Medical Center, clinical instructor at the University of Massachusetts Medical School in Worcester, and co-host of FOAMcast.
Dr. Westafer (@Lwestafer) is an attending physician and research fellow at Baystate Medical Center, clinical instructor at the University of Massachusetts Medical School in Worcester, and co-host of FOAMcast.
References
- Metlay JP, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67.
- Pastores SM, et al. Corticosteroid guideline task force of SCCM and ESICM. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2018;44(4):474–7.
- Dequin P-F, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med [Internet]. 2023. Accessed May 13, 2023.
- Andre C. Kalil, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111.
- Meduri GU, et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009–23.
Pages: 1 2 3 | Multi-Page




No Responses to “Two Big Changes in Community Acquired Pneumonia Treatment”