Explore This Issue
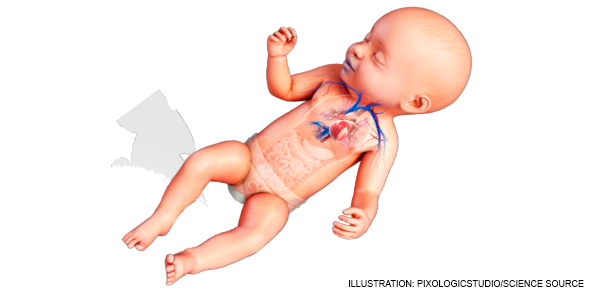
ACEP Now: Vol 37 – No 05 – May 2018The traditional approach to congenital heart disease (CHD) involves a detailed understanding of the pathophysiology, clinical findings, and management of each particular congenital heart defect. However, this cognitive-heavy approach is not practical for the emergency physician faced with an undifferentiated, unstable infant when decision making must be rapid. Despite improved CHD screening in recent years, a small but significant minority of these patients will be undiagnosed when they present to the emergency department.
In this EM Cases column, a simple approach is outlined, allowing the emergency physician to focus on time-sensitive, lifesaving treatments and practical management of the acutely ill infant with CHD.
The Three-Step Approach1
- Age: Younger than or older than 1 month? Any infant younger than 1 month old with central cyanosis or shock should be considered to have critical duct-dependent CHD until proven otherwise. This is almost always a left heart lesion such as tetralogy of Fallot, which almost always benefits from prostaglandins. Shunting or mixing lesions such as ventricular septal defect (VSD) or patent ductus arteriosus (PDA) typically present later during infancy, usually after 1 to 6 months of age.
- Color: Do they appear pink, gray, or blue? Infants with undiagnosed CHD usually present to the emergency department in one of three ways:
- Pink: Pink-appearing infants with CHD who present to the emergency department with dyspnea should have underlying acute congestive heart failure (CHF) near the top of the differential diagnosis. They have adequate pulmonary blood flow and are relatively well perfused and oxygenated. Heart failure in these patients usually occurs due to a shunting lesion. Always consider CHF in a wheezing pink child. The most sensitive and specific clinical findings for acute CHF in infants include: 1) less than 3 ounces of formula per feed (or greater than 40 minutes per breast feed); 2) a respiratory rate greater than 60 breaths per minute (or irregular breathing); and 3) hepatomegaly.2 Other clues include poor weight gain and ventricular hypertrophy on ECG.
- Gray: Gray-appearing infants with CHD are usually in shock with circulatory collapse due to poor systemic flow and oxygenation due to a left-side obstructive, duct-dependent lesion. These patients will almost always benefit from fluid administration and, if younger than 1 month in age, prostaglandins.
- Blue: The blue appearance of central cyanosis (ie, blue discoloration of the tongue, mucous membranes, and lips) in the setting of CHD usually occurs due to a right-side obstructive duct-dependent lesion in the first month of life or a mixing lesion after one month of life. These infants, like the gray ones, almost always require prostaglandins. There are four important etiologies to always consider in infants with central cyanosis: 1) CHD; 2) sepsis; 3) respiratory disorders (such as pneumonia); and 4) hemoglobinopathies (such as polycythemia and methemoglobinemia).
- Physical Examination and Bedside Tests Observing the following can provide important clues to the underlying diagnosis: the infant’s work of breathing, limb-pulse differentials, blood pressure and pulse oximetry, hyperoxia test results (see below), ECG for left ventricular hypertrophy (LVH) or right ventricular hypertrophy (RVH), and bedside cardiac ultrasound for global cardiac function, septal defects, and chamber count. After determining the infant’s color, the most important clue to CHD observed from the foot of the bed on physical exam is silent tachypnea. Tachypnea with increased work of breathing is usually due to a respiratory cause. In contrast, tachypnea without increased work of breathing—ie, silent tachypnea—is usually secondary to metabolic acidosis from a cardiac or metabolic cause.
In addition to silent tachypnea, the hyperoxia test helps differentiate respiratory causes from cardiac causes of tachypnea.3 This test was originally described using the PaO2 garnered from the arterial blood gas. Although accurate, this is a cumbersome, painful, and lengthy process.
A simpler modified method involves using the pulse oximeter before and after the patient receives 100 percent oxygen (or as close to a 100 percent FiO2 as possible) for five to 10 minutes and noting whether the oxygen saturation improves. If the oxygen saturation improves, the underlying cause of the oxygen desaturation favors a respiratory etiology. But if the oxygen saturation does not improve, a cardiac cause is more likely.
Proceed with caution when administering the hyperoxia test. Oxygen is a potent pulmonary vasodilator and could worsen respiratory distress in a patient with a duct-dependent lesion by decreasing pulmonary vascular resistance (PVR) and increasing pulmonary blood flow, leading to pulmonary over-circulation.
There are three physical exam maneuvers to consider involving limb-pulse differentials: 1) a pulse delay between radial (preductal) and femoral (postductal) pulses (or absence of femoral pulses); 2) a blood pressure differential between the right upper and a lower extremity; and 3) a difference in pulse oximetry of more than 3 percent between the right upper and a lower extremity. These all suggest a duct-dependent lesion.4
Pediatric ECG interpretation can prove challenging for many community physicians. A simple approach with regard to CHD involves the presence or absence of ventricular hypertrophy. The ECG can suggest CHD if there is evidence of LVH at any age or RVH after 1 month of age.5,6 It’s important to note that normal newborns exhibit high right-side pressures with right axis deviation and signs of RVH on the ECG. However, persistent high right-side pressures and RVH after 1 month of age is likely due to a cardiac obstructive lesion.
If you have acquired advanced cardiac ultrasound skills, bedside ultrasound may offer some clues to the underlying diagnosis in an acutely ill CHD patient.7 Ask yourself three simple questions:
- Is the global cardiac function poor (a sign of heart failure)?
- Are there four chambers of the heart (some congenital cardiac lesions involve the absence of one or more cardiac chambers)?
- Is the septum intact (consider VSD and PDA)?
Treatment Considerations
When presented with gray or blue infants suspected of duct-dependent lesions in your emergency department, CHD should be in your differential diagnosis, but it is important to remember sepsis is far more common, and as such, early empiric antibiotics should be started as soon as possible. Start prostaglandin therapy for all acutely ill gray or blue infants younger than 1 month of age to keep the PDA open. Be prepared to intubate and resuscitate the neonate who receives prostaglandins because prostaglandins can cause apnea as well as severe hypotension.
Be judicious with fluids and oxygen. Consider 5–10 mL/kg normal saline boluses rather than the usual 20 mL/kg boluses in the hemodynamically unstable infant to improve preload and encourage further opening of the PDA and pulmonary blood flow through the duct.8 Although the pulse oximetry goal in non-CHD patients is greater than 92 percent, in some CHD patients, aiming for this high of an oxygenation can prove deleterious to pulmonary blood flow, and it can worsen hypoxemia. Some CHD patients require a pulse oximetry of only 75 to 85 percent. Inotropes and/or vasopressors may be necessary to maintain adequate systemic perfusion and encourage pulmonary perfusion—consider them in consultation with a pediatric intensivist.
Avoid ketamine in patients suspected of CHD because it increases systemic vascular resistance (SVR), which worsens left-to-right shunting and can lead to cardiovascular collapse. Etomidate is preferred to minimize changes in hemodynamics.9
Be judicious with positive-pressure ventilation. Start with a very low positive end-expiratory pressure (PEEP). Positive-pressure ventilation can increase PVR and decrease SVR as well as preload, which can adversely affect shunt flow.10
Summary
The next time you’re faced with a crashing infant, rather than racking your brain for the details of every congenital heart lesion, simply consider CHD in your differential diagnosis, whether the infant is younger than 1 month of age or not, and whether they appear pink, gray, or blue. Look for silent tachypnea and assess limb differentials. Complete a hyperoxia test. Look for signs of ventricular hypertrophy on ECG. Use bedside ultrasound to determine global cardiac function, the presence of a septal defect, and the chamber count. Be judicious with fluids, oxygen, and positive-pressure ventilation. Consider prostaglandins for all blue and gray neonates, and avoid ketamine.
If you remember these simple principles, you could save an infant’s life.
Special thanks to Dr. Gary Joubert and Dr. Ashley Strobel for their contributions to the podcast from which this article was inspired.
References
- Strobel AM, Lu le N. The critically ill infant with congenital heart disease. Emerg Med Clin North Am. 2015;33(3):501-518.
- Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29(12):1535-1552.
- Mahle WT, Newburger JW, Matherne GP, et al. Role of pulse oximetry in examining newborns for congenital heart disease. A scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447-458.
- Lee JY. Clinical presentations of critical cardiac defects in the newborn: Decision making and initial management. Korean J Pediatr. 2010;53(6):669-679.
- O’Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram. Part I: Age-related interpretation. Am J Emerg Med. 2008;26(4):506-512.
- O’Connor M, McDaniel N, Brady WJ. The pediatric electrocardiogram. Part III: Congenital heart disease and other cardiac syndromes. Am J Emerg Med. 2008;26(4):497-503.
- Pershad J, Chin T. Early detection of cardiac disease masquerading as acute bronchospasm: The role of bedside limited echocardiography by the emergency physician. Pediatr Emerg Care. 2003;19(2):E1-3.
- Ricci Z, Iacoella C, Cogo P. Fluid management in critically ill pediatric patients with congenital heart disease. Minerva Pediatr. 2011;63(5):399-410.
- Zuckerbraun NS, Pitetti RD, Herr SM, et al. Use of etomidate as an induction agent for rapid sequence intubation in a pediatric emergency department. Acad Emerg Med. 2006;13(6):602-609.
- Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80(5):475-480.
Pages: 1 2 3 4 | Multi-Page






4 Responses to “A 3-Step Approach for Infants with Congenital Heart Disease”
June 7, 2018
Hexham khaledExcellent
February 25, 2019
Iman Ali Ba-SaddikA simplified approach which serves as a very good practical guide to congenital heart disease
July 5, 2019
Sunita Harkar ShallaWell written and very informative.
July 22, 2020
AmeenVery informative, Thank you so much.