Explore This Issue
ACEP Now: Vol 34 – No 03 – March 2015In 2013, ACEP updated its clinical policy for the use of intravenous tissue plasminogen activator (tPA) for the management of acute ischemic stroke in the emergency department.1 This statement, eight years in the making, was published jointly by ACEP and the American Academy of Neurology (AAN) and endorsed by the Emergency Nurses Association and the Neurocritical Care Society.
Now it’s toast.
The ensuing outcry following its publication, followed by an ACEP Council resolution to reconsider the content, has led to a wholesale revision. Most important, even more than the proposed changes to this policy, were the changes to the clinical policy process, with improved adherence to rating methodology, an open comment period to draft policies, and improved management of conflict-of-interest (COI) issues. The last issue, management of COI, was a substantial source of prior controversy, covered in part by an investigative piece about untrustworthy guidelines in The BMJ.2 The authorship of this new version of the tPA policy has changed, and any association with the AAN is conspicuously absent.
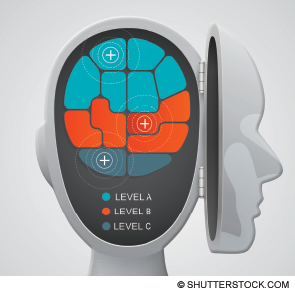
The changes enshrined in this draft are substantial. The 2013 version made two recommendations regarding the use of IV tPA in the emergency department. The first, a Level A recommendation reflecting a high degree of clinical certainty, recommended tPA be offered to ischemic stroke patients meeting National Institute of Neurological Disorders and Stroke (NINDS) criteria who are treatable within 3 hours. The second, a Level B recommendation reflecting moderate clinical certainty, recommended tPA be offered to patients meeting European Cooperative Acute Stroke Study (ECASS) III criteria who are treatable between 3 and 4.5 hours. A caveat provided for this second recommendation noted the US Food and Drug Administration license for tPA is limited to 3 hours, with the subsequent application for extension having been rejected.
The changes enshrined in this draft are substantial. The 2013 version made two recommendations regarding the use of IV tPA in the emergency department.
Many emergency physicians felt these recommendations placed them in a difficult position by endorsing a treatment with significant adverse effects. While stroke neurologists and the American Heart Association forged ahead, with substantial contributions from Genentech, a vocal cohort of emergency physicians continued to express reservations and call for more data. In March 2014, the Australasian College for Emergency Medicine outlined a position statement indicating tPA was a potentially beneficial treatment for stroke but such treatment could not be considered a standard of care in light of conflicting evidence.3 Then, in September 2014, the UK Medicines and Healthcare Products Regulatory Agency reopened a review of the “balance of benefits and risks” of the use of tPA for stroke.4 Now this new tPA policy draft shifts ACEP in the same direction.
Rather than giving treatment with tPA the Level A recommendation in this go-around, there is a new Level A recommendation (requiring high clinical certainty). It concerns the greatest fears of treatment with tPA, the risk of intracerebral hemorrhage (ICH):
“The increased risk of symptomatic intracerebral hemorrhage (approximately 7 percent compared to a baseline of 1 percent) must be considered when deciding whether to administer IV tPA to acute ischemic stroke patients.”
Essentially, based on a systematic review of randomized trials and observational registry data, the only consistent finding suitable for a Level A recommendation was a recognition of the serious adverse effects of systemic thrombolysis.
Treatment with tPA within 3 hours now becomes a Level B recommendation:
“With a goal to improve functional outcomes, IV tPA may be given to carefully selected acute ischemic stroke patients within 3 hours after symptom onset at institutions where systems are in place to safely administer the medication.”
Along with the downgrade in strength of the recommendation, the language also addresses the systems necessary to administer tPA. One safety concern shared by many emergency physicians stems from the generalizability of trial and registry data collected at dedicated stroke centers staffed by stroke neurologists. Many practice settings do not have access to the same level of subspecialty, radiology, and nursing expertise as the centers conducting stroke trials, resulting in less-safe conditions for treatment.
Use of tPA in the 3–4.5-hour time frame remains a Level B recommendation:
“Despite the known risk of symptomatic intracerebral hemorrhage and the variability in the degree of benefit in functional outcomes, IV tPA may be given to carefully selected acute ischemic stroke patients within 3 to 4.5 hours after symptom onset at institutions where systems are in place to safely administer the medication.”
Finally, the clinical policy authors added a new Level C recommendation, based on expert consensus, suggesting patients must be included in the decision-making process:
“Shared decision-making between the patient (and/or their surrogate) and a member of the health care team must include a discussion of potential benefits and harms prior to the decision whether to administer IV tPA for acute ischemic stroke.”
While this ultimate recommendation seems simply like sound, ethical practice, there are several publications from the stroke neurology literature suggesting patients ought be given tPA under “implied consent” as the “standard of care.” This recommendation reinforces the obvious necessity of a full patient and surrogate involvement when administering a medication with potentially devastating consequences. In other words, informed consent should be obtained for tPA administration.
Guidelines ought to accurately reflect the strength of the evidence, not the collective wishes and hopes of a small cohort of experts. Happily, this new version makes profound strides in sticking to appropriate grading of the evidence.
It should be noted, however, the clinical policy summarized here is only a draft, open for feedback to all concerned parties, as part of the new writing process. In general, it is a laudable effort regardless of one’s personal stance regarding tPA in acute ischemic stroke. Guidelines ought to accurately reflect the strength of the evidence, not the collective wishes and hopes of a small cohort of experts. Happily, this new version makes profound strides in sticking to appropriate grading of the evidence. That said, there are a handful of aspects in which this policy could potentially be improved:
- The policy statement describes shared decision-making and cites two examples of information graphics potentially usable for illustration of the risks and benefits. However, it is very clear from stroke trials the risk-benefit ratio differs depending on many factors, including stroke severity, specific stroke syndromes, and individual patient substrate. Several models have attempted to individualize the risk of symptomatic ICH compared to baseline with uncontrolled diabetes, uncontrolled hypertension, and age the most important predictors. The policy statement alludes to a need for further research necessary to tailor treatment to the individual patient but understates this critical need. Considering it has been 20 years since the original NINDS trial, still having inadequate evidence with which to guide decision-making is nonsensical. This document could be a powerful platform with which to state clinical equipoise and call for additional placebo-controlled trials.
- The Level B recommendation for the 3–4.5-hour time window is difficult to justify based on the stated recommendation criteria. The authors rate the one positive study, ECASS III, as Class II evidence based on potential for bias. Class III data from ATLANTIS and IST-3, however, provide negative data. ATLANTIS was modified several times (including due to safety monitoring) before ultimately settling on a 3–5-hour time window and was then stopped early for futility. IST-3 suffered from an open-label design but was particularly unfavorable within the 3–4.5-hour window, with 31.5 percent having good outcomes given tPA compared with 37.7 percent in the control group. The individual-patient meta-analysis further cited in support of the 3–4.5-hour window derives most of its patients from these three trials, providing limited additive information. This probably does not meet their stated criteria for a recommendation with “moderate clinical certainty.”
- Several statements use vague terminology to describe the specifics of treatment. Each recommendation for tPA use mentions “carefully selected patients” and “systems in place to safely administer the medication.” If the recommendations propose strict adherence to ECASS III or ECASS III enrollment criteria, this should be clearly stated. Likewise, if “systems in place” refers to a certification such as The Joint Commission Advanced Comprehensive Stroke Center, this should also be clarified.
- Ultimately, even though the document explicitly states it is not intended to represent a legal standard of care for emergency physicians, it will certainly be wielded as such to either protect or crucify. To that end, it could use language specifically protecting the clinician who chooses not to offer tPA, in recognition of the persistent uncertainty responsible for the downgrading of recommendations from Level A.
But these are only my initial reactions, subject to the limitations of my own knowledge base and biases. Luckily, this document was open to feedback from all emergency physicians. The comment period closed March 13, 2015. The final policy should be forthcoming.
I hope you made your opinions known!
 Dr. Radecki is assistant professor of emergency medicine at The University of Texas Medical School at Houston. He blogs at Emergency Medicine Literature of Note (emlitofnote.com) and can be found on Twitter @emlitofnote.
Dr. Radecki is assistant professor of emergency medicine at The University of Texas Medical School at Houston. He blogs at Emergency Medicine Literature of Note (emlitofnote.com) and can be found on Twitter @emlitofnote.
References
- American College of Emergency Physicians; American Academy of Neurology. Clinical policy: use of intravenous tPA for the management of acute ischemic stroke in the emergency department. Ann Emerg Med. 2013;61:225-43
- Lenzer J. Why we can’t trust clinical guidelines. BMJ. 2013;346:f3830.
- Australasian College for Emergency Medicine. S129 statement on intravenous thrombolysis for ischaemic stroke. Updated March 2014.
- Cohen D, Macdonald H. UK drug agency announces review of use of alteplase after stroke. BMJ. 2014;349:g5355.
Pages: 1 2 3 | Multi-Page




2 Responses to “ACEP Clinical Policy on Intravenous Tissue Plasmogen for Stroke Continues to Evolve”
March 18, 2015
Brian S. AlperFor a detailed analysis of the evidence on t-PA 3-4.5 hours after stroke see BMJ 2015;350:h1075 published March 17 at http://www.bmj.com/content/350/bmj.h1075
We shared this information during the draft guideline feedback period so hopefully it will be analyzed while the policy-making is in full swing — harder to adjust after formal publication.
We also shared with CAEP (guideline in draft state with weak recommendation against) and MHRA (the UK drug regulating agency reconsidering drug licensing)
May 18, 2015
The tPA controversy | Sinai-Grace Emergency Medicine Residency[…] of ACEP Now (it’s that monthly newsletter that we all get and occasionally read). Here is the link. Essentially, the clinical guidelines recommending tPA are changing. They used to be level A […]