A 3-year-old male was brought to the emergency department (ED) by his mother, who reported the sudden onset of a rash (hives) covering his entire body, with no rash on his palms and soles. No other complaints were noted. The child’s skin appeared warm and dry. A review of systems revealed no abnormal findings. Vitals were within normal limits, with a pulse of 129, respiratory rate of 25, and oxygen saturation of 98 percent. On physical examination, the child was non-toxic, well-nourished, alert, awake, and not in acute distress. The child was diagnosed with hives and discharged to home with symptomatic management.
Explore This Issue
ACEP Now: Vol 43 – No 01 – January 2024The next day, the patient’s mother was called to come to the emergency department with the patient due to abnormal labs. The patient had an abnormal lead screening test at age 2 and was advised to receive a comprehensive lead screening evaluation, which he did approximately one year later, shortly prior to this ED visit. Incidentally, the day after ED visit number one, the mother was called by the child’s pediatrician advising her to bring him for evaluation of lead poisoning. The initial laboratory results were abnormal, with a lead level of 56.9 μg/dL, hemoglobin of 6.4 g/dL, hematocrit of 23.9 percent, mean corpuscular volume of 53 fL, mean corpuscular hemoglobin of 14 pg, mean corpuscular hemoglobin concentration of 26.4 g/dL, and red cell distribution width of 20.84 percent.

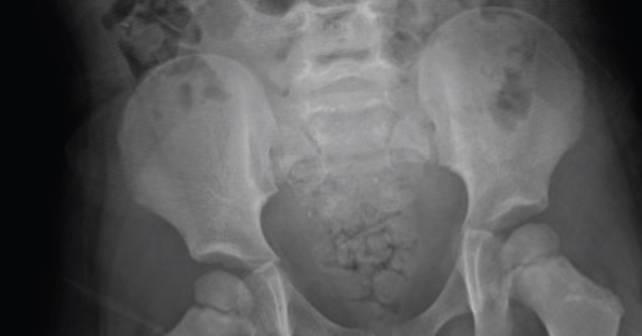
FIGURE 1: Abdominal X-ray showing particulate radiopaque foreign bodies involving the stool. (Click to enlarge.)
A peripheral smear revealed mild anisocytosis, ovalocytes, hypochromia, microcytosis, and poikilocytosis. Blood type and screen, COVID-19 tests, and ECG were all within normal limits. An abdominal X-ray revealed particulate radiopaque foreign bodies involving the stool. The mother revealed a history of elevated blood lead levels when the child was two years old, indicating a previous exposure. The family had since relocated to a lead-safe environment. In the emergency department, consultations were made with the regional poison-control center and the regional lead center. A decision was made to transfer the patient to a tertiary center with inpatient pediatric capabilities. The patient was subsequently transferred to another medical center for treatment of anemia and lead chelation.
Discussion
Lead poisoning is a serious health concern. It can present in acute and chronic forms. It can occur due to accidental ingestion or occupational or environmental exposure.1 Children are particularly vulnerable.2 According to the latest definitions from the Centers for Disease Control and Prevention, blood lead concentrations equal to or exceeding 5 μg/dL are classified as elevated levels in both adults and children.3-5 Exposure to lead in humans can occur through a range of sources, encompassing lead-based paints, leaded gasoline, lead-containing pipes, lead smelting, coal combustion, and occupational activities like battery recycling.6 Lead poisoning primarily occurs through two main routes: ingestion and inhalation. Ingestion is more prevalent among children due to their inclination to put objects in their mouths, whereas inhalation is a more common route of exposure in occupationally exposed adults.7 The human body can store lead in specific tissues, including bones, teeth, hair, and nails, where it forms tight bonds and appears to be relatively inert, posing less immediate harm as it is less available to affect other bodily tissues.8 Interestingly, in children, approximately 70 percent of the absorbed lead accumulates in their bones, whereas in adults, a higher proportion, around 94 percent, is deposited there. This difference in lead distribution may contribute to the more pronounced clinical effects of lead poisoning in young children.9 Lead exerts its toxic effects by interfering with various organ functions, primarily targeting the nervous system and hematopoietic system, as well as impairing liver and kidney functions.3
Lead poisoning occurs most commonly in the developing world.10 There have also been numerous cases in the developed world, with higher lead burdens seen during the peak of the Flint, Michgan, water crisis.11 Lead interacts with human physiology in two significant ways: it strongly binds to sulfhydryl groups and other electron donor groups in proteins, affecting their functions. Additionally, its similarity to divalent cations like calcium and zinc disrupts various cellular processes regulated by these ions.12 From a neurological standpoint, lead is believed to disrupt the natural pruning of synapses in developing brains, which may explain cognitive and behavioral changes observed in children exposed to high levels of lead.13 Peripheral neuropathy is a common manifestation of lead toxicity in adults but its mechanism is poorly understood. Severe neurological manifestations seen in lead encephalopathy are thought to be at least in part due to lead-induced cerebral microvascular changes leading to cerebral edema and increased intracranial pressure.14 Lead-induced anemia occurs because it disrupts enzymes responsible for making heme and maintaining red blood cell membranes. This disruption reduces production of red blood cells and increases their destruction.12 From a kidney standpoint, lead can cause problems in the proximal tubules, similar to Fanconi syndrome, and it competes with uric acid for excretion in the distal tubule, raising blood urate levels. Lead also has a multitude of effects on the endocrine system, thyroid function, skeletal growth, and development.15 Lead is associated with gastrointestinal symptoms such as abdominal pain, constipation, and anorexia but these effects are poorly understood.12 Our patient exhibited hematological manifestations of lead poisoning, but on examination was asymptomatic apart from a rash on initial presentation.
Chelation therapy is recommended for severe lead poisoning based on age, blood lead concentration, and clinical symptoms.16 Chelation therapy is recommended for patients with blood lead concentrations exceeding 45 μg/dL.17 In the past, a combination of dimercaprol and calcium disodium ethylenediaminetetraacetic acid (abbreviated EDTA) was the recommended chelation regimen. However, today, dimercaptosuccinic acid (aka DMSA or succimer) is approved and recommended for these patients. For those with mild to moderately increased lead levels, D-penicillamine was previously used orally but, due to toxic effects, has largely been replaced by succimer since 1991.18
Conclusion
Our case highlights the importance of vigilant lead screening in young children, even in the absence of overt symptoms. Timely identification of elevated lead levels and appropriate intervention can prevent the detrimental effects of lead poisoning. Emergency physicians should remain vigilant in identifying potential sources of lead exposure in at-risk populations.
 Dr. Cohen is the medical director and chairman of Emergency Services at Montefiore St. Luke’s Cornwall in Newburgh, NY.
Dr. Cohen is the medical director and chairman of Emergency Services at Montefiore St. Luke’s Cornwall in Newburgh, NY.
 Dr. Wazir is an ECFMG-certified international medical graduate from Pakistan. Dr. Wazir is currently applying for a residency position in emergency medicine.
Dr. Wazir is an ECFMG-certified international medical graduate from Pakistan. Dr. Wazir is currently applying for a residency position in emergency medicine.
References
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47.
- Blank E, Howieson J. Lead poisoning from a curtain weight. JAMA. 1983;249(16):2176-7.
- Nakhaee S, Amirabadizadeh A, Brent J, et al. Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Pharmacol Toxicol. 2019;124(5):621-8.
- Ruckart PZ, Jones RL, Courtney JG, et al. Update of the blood lead reference value—United States, 2021. MMWR. 2021;70(43):1509.
- Zardast M, Khorashadi-Zadeh SS, Nakhaee S, et al. Blood lead concentration and its associated factors in preschool children in eastern Iran: a cross-sectional study. BMC Pediatr. 2020;20(1):435.
- Tong S, Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78(9):1068-77.
- Dapul H, Laraque D. Lead poisoning in children. Adv Pediatr. 2014;61(1):313-33.
- Rubin R, Strayer DS, Rubin E, eds. Rubin’s pathology: clinicopathologic foundations of medicine. Baltimore: Lippincott Williams & Wilkins;2008:307-9.
- Barbosa Jr F, Tanus-Santos JE, Gerlach RF, et al. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ Health Perspect. 2005;113(12):1669-74.
- World Health Organization. The public health impact of chemicals: knowns and unknowns. Geneva: World Health Organization;2016:9-12.
- Roy S, Edwards MA. Preventing another lead (Pb) in drinking water crisis: Lessons from the Washington DC and Flint MI contamination events. Curr Opin Environ Sci Health. 2019;7:34-44.
- Mitra P, Sharma S, Purohit P, Sharma P. Clinical and molecular aspects of lead toxicity: An update. Crit Rev Clin Lab Sci. 2017;54(7-8):506-28.
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(1):5-19.
- de Souza A, Narvencar KP, Desai PK, et al. Adult lead encephalopathy. Neurol Res. 2013;35(1):54-8.
- Halmo L, Nappe TM. Lead toxicity. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2023. Last updated July 4, 2023. Accessed December 13, 2023.
- Cantor AG, Hendrickson R, Blazina I, et al. Screening for elevated blood lead levels in childhood and pregnancy: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(15):1510-26.
- Bradberry S, Vale A. Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning. Clinical Toxicology. 2009;47(7):617-31.
- Samarghandian S, Shirazi FM, Saeedi F, et al. A systematic review of clinical and laboratory findings of lead poisoning: lessons from case reports. Toxicol Appl Pharmacol. 2021;429:115681.
Pages: 1 2 3 4 | Multi-Page




No Responses to “Case Report: A Child with a Rash and Lead Poisoning History”