A 10-year-old male with a past medical history significant for autism spectrum disorder and 15q3 deletion, experienced a cardiac arrest at home. There was no family history of syncope or sudden death. EMS found the patient pulseless and apneic, with an initial rhythm showing ventricular fibrillation (see figure 1). He was defibrillated twice and received two doses of epinephrine, with return of spontaneous circulation.
Explore This Issue
ACEP Now: Vol 43 – No 10 – October 2024Initial echocardiogram (ECG) on arrival (see figure 2) to our emergency department revealed normal sinus rhythm, mild interventricular conduction delay (RSR’), and possible right ventricular hypertrophy. This ECG in combination with presenting symptom of cardiac arrest raised suspicion for Brugada syndrome.
A repeat ECG (see figure 3) was performed utilizing the technique described by Sangwatanaroj et al., with right precordial lead modification, moving lead V3 one intercostal space above V1 and V4 one space above V2 (third intercostal space).1,2 This ECG showed Brugada pattern with coved ST-segment and J point elevation in V1 and V2.
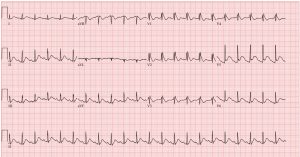
Figure 3: An ECG obtained with modified “high” lead placement confirming Brugada syndrome. (Click to enlarge.)
Pediatric cardiology was consulted. His ECG demonstrated normal biventricular systolic function, mildly elevated pulmonary pressures, and a structurally normal heart. He underwent placement of a dual chamber, implantable, cardioverter-defibrillator (ICD) placement on hospital day 5. At discharge, his neurological insult was severe, with significantly impaired physical and cognitive function, aspiration of feeds, and visual impairment.
Discussion
Brugada syndrome is an inherited disorder, due to a sodium channelopathy, characterized by right ventricular conduction delay, “coved” ST-segment elevation in the right precordial leads (V1-V3), and an increased risk of syncope and sudden cardiac death.3-6 Additional features include first-degree atrioventricular block, atrial arrhythmias, nocturnal agonal breathing, and seizures. Arrythmias are common during febrile illnesses and in sleep.

Figures 4:
A. Type 1 is diagnostic and characterized by J point and ST elevation > 2 mV with coved ST- elevation in at least two precordial leads, followed by negative T wave.
B. Type 2 is characterized by elevated J point and elevated ST ≥ 1 mm, saddle-shaped, flat and followed by positive T wave.
C. Type 3 is indicated by J point and ST elevation < 1 mm.
(Click to enlarge.)
The different morphology of Brugada is shown in Figure 4; although, only type 1 pattern is diagnostic.5,7,8 The ECG patterns of Brugada derive from the conduction delay localized in the right ventricular anterior wall and right ventricular outflow tract. By moving the anterior precordial leads higher on the chest to the second or third intercostal spaces, a Brugada pattern can be brought out (see figure 5).9
It is estimated that 20 percent of sudden cardiac deaths with a structurally normal heart are attributed to Brugada syndrome.10 The condition is autosomal dominant with variable expression, more common in males, with low prevalence in children (<0.8 percent).7 The ECG findings can be concealed or transient after resuscitation from cardiac arrest. Many cases are asymptomatic, and ECG changes may only be seen intermittently during conditions like fever, vagal stimulation, drug intoxication, or electrolyte imbalances. The absence of findings between episodes makes this diagnosis elusive.
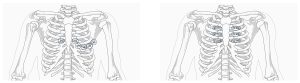
Figure 5: This graphic shows a comparison of traditional and modified location of placement of right precordial leads. (Click to enlarge.)
The most common presenting symptoms are life-threatening arrhythmias, syncope, and cardiac arrest. Diagnosis may be made based on ECG findings and the association of one of the following: documented ventricular fibrillation, polymorphic ventricular tachycardia, family history of Brugada syndrome, sudden unexplained death in family members younger than 45 years old, unexplained syncope or aborted sudden death, as in our case.11 The procainamide challenge test can also be used to unmask latent Brugada syndrome in asymptomatic individuals.12
In children, an ICD is considered a class 1 indication in patients with aborted sudden cardiac death or spontaneous, sustained ventricular tachycardia. Fever and certain drugs can precipitate arrhythmias in this population. A list of medications to avoid in Brugada syndrome are available at https://www.brugadadrugs.org/avoid/. Genetic testing may inform prognosis and management, as certain SCN5A mutations are associated with a higher risk of arrhythmic events; although, the yield of genetic testing is fairly low in this condition.13
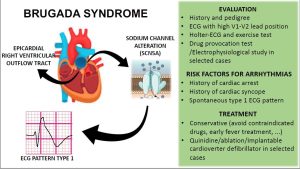
Figure 6: A summary graphic of diagnosis and management of Brugada syndrome in pediatrics. (Click to enlarge.)
A summary approach to diagnosis and management of pediatric patients with suspected Brugada syndrome is shown in Figure 6.
Key Takeaways for Emergency Physicians
- Brugada syndrome is an uncommon cause of sudden cardiac arrest in children. It must be suspected in aborted sudden cardiac death or unexplained syncope in the presence of conduction delay and repolarization abnormalities in precordial leads. It can present as asystole or ventricular fibrillation.
- ECG findings may be concealed or seen intermittently in association with conditions like fever, vagal stimulation or electrolyte imbalances.
- Modified lead placement, with high precordial leads at the second or third intercostal space superior may help confirm diagnosis.
A special thanks to Dr. Elizabeth Sherwin, Pediatric Cardiologist & Electrophysiologist at Children’s National Hospital.
Dr. Pershad is an emergency medicine physician at Children’s National Hospital, Washington, D.C.
Dr. Lacey is a pediatric cardiologist at Children’s National Hospital, Washington D.C.
References
- Sangwatanaroj S, Prechaway S, Sunsaneewitayakul B, et al. Right ventricular electrocardiographic leads for detection of Brugada syndrome in sudden unexplained death syndrome survivors and their relatives. Clin Cardiol. 2001;24(12):776-781.
- Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, et al. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22(24):2290-2296.
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111(5):659-670.
- Probst V, Denjoy I, Meregalli PG, et al. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115(15):2042-2048.
- Speranzon A, Chicco D, Bonazza P, et al. Brugada Syndrome: Focus for the General Pediatrician. Children (Basel). 2024;11(3):281.
- El-Battrawy I, Roterberg G, Schlentrich K, et al. Clinical Profile and Long-Term Follow-Up of Children with Brugada Syndrome. Pediatr Cardiol. 2020;41(2):290-296.
- Gonzalez Corcia MC, de Asmundis C, Chierchia GB, Brugada P. Brugada syndrome in the paediatric population: a comprehensive approach to clinical manifestations, diagnosis, and management. Cardiol Young. 2016;26(6):1044-1055.
- Peltenburg PJ, Hoedemaekers YM, Clur SAB, et al. Screening, diagnosis and follow-up of Brugada syndrome in children: a Dutch expert consensus statement. Neth Heart J. 2023;31(4):133-137.
- Krah AD, Behr ER, Hamilton R, et al. Brugada Syndrome. JACC Clin Electrophysiol. 2022; 8(3):386-405.
- Kabra N, Gupta R, Aronow WS, Frishman WH. Sudden Cardiac Death in Brugada Syndrome. Cardiol Rev. 2020;28(4):203-207.
- Brugada J, Campuzano O, Arbelo E, et al. Present Status of Brugada Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(9):1046-1059.
- Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932-1963.
- Pannone L, Bisignani A, Osei R, et al. Genetic Testing in Brugada Syndrome: A 30-Year Experience. Circ Arrhythm Electrophysiol. 2024;17(4):e012374.
Pages: 1 2 3 | Multi-Page





No Responses to “Case Report: Cardiac Arrest in a Child’s Structurally Normal Heart”