
This patient is a 52-year-old male with past medical history of hypertension and hyperlipidemia who presented to the emergency department (ED) with approximately one month of progressive bilateral ptosis and one week of progressive bilateral diplopia and photophobia. The patient reported that these symptoms were more noticeable later in the day and worsened with reading and typing. The patient’s gaze disturbance was first noted by his wife, and he agreed to be evaluated by an optometrist. The patient was seen and instructed to report to the ED for concern of neuromuscular or intracranial pathology. After an unremarkable initial workup at an outside ED, which included head CT/CTA, blood chemistry, chest radiograph, and ECG, the patient was transferred to our tertiary care center for further neurological evaluation.

IMAGE 2: Upward gaze on presentation, note frontalis muscle compensation to lift eyelids.
The patient’s vital signs were within normal limits aside from mildly elevated blood pressure. Review of systems was positive for headache, diplopia, and photophobia. He denied recent fever, seizures, vertigo, limb weakness, stiffness, or pain. Reassuringly, the patient denied having any respiratory complaints. On physical exam, the patient exhibited bilateral ptosis (see Image 1). Extraocular range of motion was intact, but he had some difficulty with vertical gaze, which was easily fatigable and accompanied with noticeable compensation by the frontalis muscle (see Image 2). Further ocular testing revealed positive accommodation but marked photophobia. This prevented pupillary light reflex testing because the patient squeezed his eyelids shut to bright light. He was unable to complete visual field testing due to diplopia. The patient’s neck exhibited full active and passive range of motion with no tenderness or meningismus.
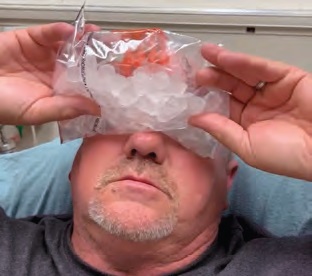
IMAGE 3: Administering the ice pack test.
The remainder of physical and neurological exams were unremarkable.
Diagnosis and Initial Treatment
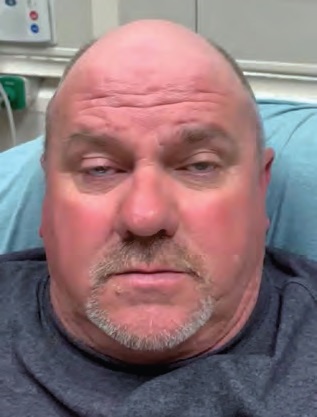
The decision was then made to test for myasthenia gravis (MG) by application of an ice pack. A plastic bag was filled with ice, and the patient was instructed to hold this over his eyes for two minutes (Image 3). The ice pack was then removed, and both patient and examiners observed noticeable improvement. The patient’s ptosis was absent (Image 4). Extraocular range of motion was normal (Image 5), and the patient reported resolution of diplopia and photophobia. Pupillary light reflex was assessed and found to be normal. This effect lasted for approximately 90 seconds before ptosis reappeared. The test was conducted twice, approximately 15 minutes apart, with identical results, and the diagnosis of MG was made.

IMAGE 4: Forward gaze after ice pack test.
The patient was admitted to the hospital for continued neurological workup. Blood samples were sent to an outside lab to check for the presence of anti-acetylcholine (AcH) and anti-muscle-specific tyrosine kinase (MuSK) antibodies. Soon after arriving to the floor, the patient was evaluated by the staff neurologist, who agreed with our diagnosis and ordered prednisone and pyridostigmine. A chest CT demonstrated no thymic or respiratory abnormalities, and brain MRI was unremarkable. Approximately 24 hours after first arriving in the ED, the patient reported that his symptoms had greatly improved and exhibited no ptosis or vertical gaze deficit.
Discussion
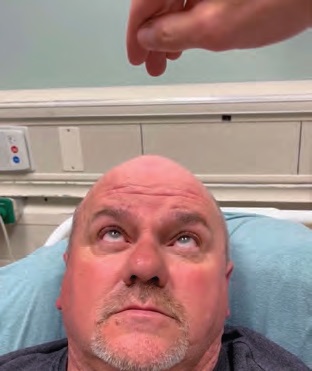
IMAGE 5: Upward gaze after ice pack test.
Myasthenia gravis is the most common disorder of neuromuscular transmission.2 The prevalence of MG in the United States is estimated to be 37 per 100,000, with estimated incidence ranging from 4.1 to 30 cases per million person-years.3,4 The disorder most commonly initially presents in females more than 40 years old and men over the age of 60. MG may present at any age in either sex, although it is rare in children.3 MG has a strong association with thymic hyperplasia and thymoma. It is estimated that 13 percent of patients with MG have one or more coexisting autoimmune diseases, most commonly affecting the thyroid gland. The estimated rate of co-occurrence is 7 percent with Graves’ disease and 3 percent with Hashimoto’s thyroiditis, which may contribute to symptoms of ophthalmopathy and weakness if present.5
Most patients initially present with intermittent flares of ptosis, extraocular muscle weakness, and diplopia. The second most common presentation involves bulbar muscle weakness, dysphagia, dysarthria, and frequent aspiration.6 A key feature in diagnosis is weakness that worsens with muscle use and improves with rest. Left untreated, weakness may spread and become generalized. In severe flares, known as myasthenic crises, the diaphragm and accessory muscles of breathing may become significantly weak, requiring noninvasive positive airway ventilation or intubation with mechanical ventilation. For this reason, prompt diagnosis and initiation of treatment are vital for individuals experiencing their first MG flare.
The mechanism in ptosis and in MG is believed to be the inhibition of the levator palpebrae superioris muscle at its lower motor neuron synapse with the oculomotor nerve. This is caused by the blockade of nicotinic AcH receptors by autoantibodies. Several mechanisms have been proposed to explain the temporary improvement of ocular MG symptoms after cooling.1 Although colder temperatures have been demonstrated to decrease the speed of nerve conduction, they also inhibit the action of acetylcholinesterase, resulting in a greater amount of neurotransmitter for a longer period to be present in the gap junction. This mechanism is also applied in the use of the acetylcholinesterase inhibitor pyridostigmine as first-line treatment for myasthenic flares.6,7
In addition to pyridostigmine, immunosuppression with glucocorticoids or azathioprine is indicated for acute myasthenic flares. Second-line immunosuppressive agents include methotrexate and cyclophosphamide. For patients experiencing myasthenic crisis, intravenous immunoglobulin or plasmapheresis are indicated to prevent respiratory collapse due to their rapid onset. Thymectomy is recommended in patients with evidence of thymoma, seronegative disease, or anti-nicotinic-AcH subtype of disease if presentation occurs between the ages of 15 and 50.6
The initial diagnostic workup for patients with MG symptoms should include a complete neurological exam. Additionally, cranial imaging should be used to rule out stroke or intracranial mass, which may present with similar symptoms. Patients should also undergo CT or MR imaging of the thymus, as well as measurement of thyroid-stimulating hormone to investigate concomitant autoimmune thyroid disease. Differential diagnosis may include disorders of the neuromuscular junction or the peripheral nervous system, such as Lambert-Eaton Syndrome, botulism, Horner Syndrome, neuromyotonia, or Guillain-Barré Syndrome. Diagnosis may be confirmed by nerve conduction study or by the presence of anti-AcH or anti-MuSK antibodies, although a small percentage of patients are seronegative.8 Historically, checking for symptom improvement in response to intravenous edrophonium, a short-acting acetylcholinesterase inhibitor, was seen as the gold standard diagnostic test for MG, but this has since been supplanted by serologic and nerve conduction studies due to the concern for serious side effects, including bradycardia and bronchospasm.1 The ice pack test has proven to be an effective initial method of diagnosis for ocular MG. When used to detect MG in patients presenting with diplopia, ptosis, or both, the ice pack test has been reported to demonstrate a sensitivity of 76.9 percent and a specificity of 98.3 percent.9 The authors of this study found the test to remain highly specific, even in patients with co-existing thyroid dysfunction.
Conclusion
Patients presenting with their first MG flare require prompt diagnosis and treatment to prevent progression of symptoms. The initial diagnosis of MG is largely clinical. Results from anti-AcH and Anti-MuSK serologic testing may take several days, and treatment should not be withheld for confirmatory testing. In this patient’s case, ice pack testing could have easily been done at the first ED. A correct diagnosis was made quickly at our facility, which allowed for rapid admission and initiation of treatment. With maintenance immunosuppression, the prognosis of individuals with ocular MG is good; most live a normal life expectancy with minimal disability.8
 Dr. Cray is a first-year emergency medicine resident at Summa Health in Akron, Ohio, with interests in EMS and wilderness medicine.
Dr. Cray is a first-year emergency medicine resident at Summa Health in Akron, Ohio, with interests in EMS and wilderness medicine.
 Dr. Bennett is a third year resident at Charleston Area Medical Center in Charleston, West Virginia.
Dr. Bennett is a third year resident at Charleston Area Medical Center in Charleston, West Virginia.
References
- Marinos E, Buzzard K, Fraser CL, et al. Evaluating the temperature effects of ice and heat tests on ptosis due to myasthenia gravis. Eye (Lond.) 2018;32(8):1387-1391.
- Rubin M. Disorders of neuromuscular transmission (neuromuscular junction disorders). Merck Manuals Professional Version. Reviewed/Revised March 2024. Accessed July 18, 2024.
- Bubuioc AM, Kudebayeva A, Turespekova S, et al. The epidemiology of myasthenia gravis. J Med Life. 2021;14(1):7-16.
- Dresser L, Wlodarski R, Rezania K, et al. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11):2235.
- Mao ZF, Yang LX, Mo XA, et al. Frequency of autoimmune diseases in myasthenia gravis: a systematic review. Int J Neurosci. 2011;121(3): 121-129.
- Beloor SA, Asuncion RMD. Myasthenia gravis. In: StatPearls [Internet]. Published August 8, 2023. Accessed July 18, 2024.
- Foldes FF, Kuze S, Vizi ES, et al. The influence of temperature on neuromuscular performance. J Neural Transm. 1978;43(1):27-45.
- Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2014;175(3) 408-418.
- Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116(11):2236-2243.
Pages: 1 2 3 4 | Multi-Page




No Responses to “Case Report: The Ice Pack Test”