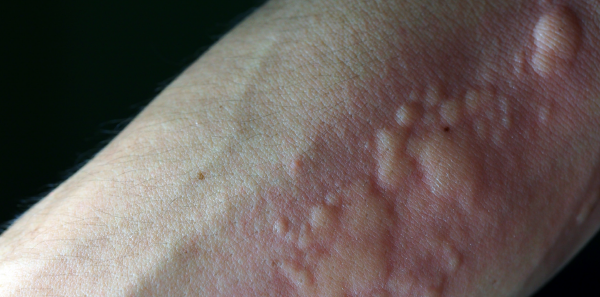
Medical news consumers may have noted the arrival of intravenous cetirizine (Quzyttir). This second-generation “nonsedating” antihistamine was approved by the U.S. Food and Drug Administration (FDA) in October 2019. Since then, its defining trial has been published in the Annals of Emergency Medicine, and promotional material now shows up in some trade magazines.1
Explore This Issue
ACEP Now: Vol 40 – No 06 – June 2021Intravenous cetirizine is precisely what you envision it to be: a 10-mg dose of cetirizine hydrochloride in sodium chloride for injection. Likewise, it operates as expected: selective inhibition of peripheral of H1 receptors. It is currently approved for treatment of acute urticaria, an indication currently treated by off-label use of the remaining family of first- and second-generation antihistamines. However, while it and its second-generation cousins are available in oral formulation, availability for intravenous injection is restricted to diphenhydramine, while hydroxyzine is available for intramuscular use. An intravenous formulation of a selective H1 inhibitor therefore has potential advantages.
The Research
These potential advantages were examined in the aforementioned randomized, double-blind, active-comparator trial conducted in 19 emergency departments and urgent care centers in the United States and Canada.1 This trial enrolled patients with acute urticaria and an elevated “patient-rated pruritis severity score,” comparing 10 mg of intravenous cetirizine with 50 mg of intravenous diphenhydramine. The primary endpoint of the trial was the patient-rated pruritis severity score at two hours following admission. If you are not already familiar with this scoring system, it is likely because it was created specifically by the trial sponsor for the purpose of this trial. This ordinal scale ranges from 0 to 3, in which 0 is “no pruritis,” 1 is “mild,” 2 is “moderate,” and 3 is “severe.” The trial was designed to test for the noninferiority of IV cetirizine compared to diphenhydramine.
The Results
Among 262 patients randomized into roughly similar cohorts, there were ultimately no differences in this patient-reported outcome measure. Each group complained of an average of “moderate” pruritis at baseline, and each displayed similar general declines in symptoms within an hour. Considering the almost identical clinical performance of each drug, the prespecified margin for statistical noninferiority was easily met.
The more interesting clinical information comes from the many secondary outcomes. Diphenhydramine, as a first-generation antihistamine, displays greater sedating and anticholinergic properties. These adverse effects can be particularly undesirable in an elderly population or in people who have to stay alert during the day or at work, for example. To this effect, the authors measured a “patient-rated sedation score” for each group, an ordinal clinical scale defined only in this trial. To little surprise, the sedation perceived by patients receiving diphenhydramine exceeded that of those given intravenous cetirizine. The mean level of sedation rose from baseline to “mild” in those receiving diphenhydramine, while those receiving cetirizine generally remained close to their baseline upon enrollment.
Other key secondary endpoints included physician assessment of urticaria, effectively treated patients, time spent at treatment centers, and short-term recidivism. The results were full of minor curiosities. Even though the patient-rated pruritis scores improved similarly for each intervention, nearly twice as many patients in the diphenhydramine group received a rescue agent of some kind. These agents were most frequently a steroid, whether prednisone, methylprednisolone, or dexamethasone. Then, oddly, despite this excess of patients receiving steroids in the diphenhydramine, short-term recidivism favored cetirizine. The reasons for follow-up visits were not reported, so it is difficult to determine whether these were related to treatment failure or other adverse effects. It is also possible, particularly in a small trial like this, that there are simply unmeasured differences between the groups.
Lastly, the authors focus on time spent at treatment centers, which is distinct from length of stay. This outcome captures the time from treatment administration to discharge rather than from emergency department or urgent care center arrival. Roughly speaking, there was an advantage to intravenous cetirizine of about 20 to 30 minutes over diphenhydramine, which has reasonable face validity. However, this difference was not statistically significant under their prespecified analysis plan, and it was found only in an ad hoc analysis designed after efficacy data were already known.
Should We Add It to Our Toolbox?
Having summarized the high-level observations from this trial, it is reasonable to conclude, as the FDA reviewers did, that IV cetirizine is safe and effective.2 The more challenging question addresses its appropriateness. The justification for its use in acute urticaria is stated by the authors as “the parenteral route of administration is often preferred,” citing a generalist American Family Physician review article that, interestingly enough, does not actually include such a statement.3 There is no evidence IV agents provide superior treatment of urticaria than oral ones for this frequent condition. In fact, the most recent practice update from the immunology community does not specify any specific route of administration for the most effective treatment of urticaria.4
To this larger point, the original oral cetirizine pharmacology labeling information from Pfizer in 2002 described rapid onset of inhibition of histamine release reaching 95 percent effectiveness within one hour.5 Furthermore, the mean area under the concentration-time curve for the IV formulation is identical to that of the oral formulation. The obvious difference between the preparations is the IV form reaches a peak concentration of >300 percent the oral formulation and within minutes rather than an hour. The FDA reviewers, rather than commenting on a potential clinical benefit, focused instead on the safety profile of such a high serum level.
The bottom-line question: Does this product have a purpose? For its approved indication of acute urticaria, the most likely answer is almost never. Absent evidence to demonstrate the advantages of IV therapy over oral, the question asked by this trial is virtually moot, other than perhaps in patients unable to tolerate oral intake. For most patients, any rescue medications, such as H2 blockers or steroids, can just as easily be given by mouth. But what about the “time in treatment center”? As noted above, measuring this outcome obscures the practicalities of a busy emergency department, particularly the resources required for IV access in the first place. A patient presenting with acute urticaria can be treated with an oral second-generation antihistamine at triage and easily reach therapeutic serum levels in less time than an intravenous version could be administered. In other words, if the study measured time from triage, most of the outcomes would likely have been indistinguishable. The authors seem to have forgotten that avoiding an IV is itself a positive outcome.
But what about anaphylaxis? In these cases, IV access is usually obtained early anyway. But the consensus from the allergy and immunology community is quite clear (though markedly different from standards of care in most EDs) that neither antihistamines nor steroids have any important role in treatment of anaphylaxis and certainly need not be given intravenously. A patient with an uncomfortable urticarial component to anaphylaxis not resolving with epinephrine is just as well-served by oral antihistamines. And those patients are not being discharged in an hour.
The final tiebreaker, of course, is always cost. The wholesale acquisition cost of Quzyttir is reportedly $300 for a 10-mg vial, which, combined with additional nursing resource charges for intravenous access and medication administration, rapidly increases the total cost for an acute encounter.6 Conversely, if prudent avoidance of therapies lacking clear added value is important, then continued routine use of generic oral second-generation antihistamines will serve just as well.
The opinions expressed herein are solely those of Dr. Radecki and do not necessarily reflect those of his employer or academic affiliates.
References
- Abella BS, Berger WE, Blaiss MS, et al. Intravenous cetirizine versus intravenous diphenhydramine for the treatment of acute urticaria: a phase III randomized controlled noninferiority trial. Ann Emerg Med. 2020;76(4):489-500.
- NDA multidisciplinary review and evaluation {NDA 211415} {Quzyttir (cetirizine hydrochloride injection) for intravenous use}. U.S. Food and Drug Administration website. Accessed May 18, 2021.
- Schaefer P. Acute and chronic urticaria: evaluation and treatment. Am Fam Physician. 2017;95(11):717-724.
- Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123.
- Zyrtec (cetirizine hydrochrloride) tablets and syrup for oral use. U.S. Food and Drug Administration website. Accessed May 18, 2021.
- Drug monograph: Quzyttir. Conduent website. Accessed May 18, 2021.
Pages: 1 2 3 | Multi-Page




2 Responses to “Does New IV Urticaria Medication Offer Benefits Over Current Treatments?”
July 19, 2021
Dr. Joseph SachterExcellent review — as usual. Although I might use the intravenous preparation a little more frequently than “almost never’ (an unaccompanied patient who drove to themselves to the ED and might otherwise be ready for discharge a couple of hours after treatment initiation), the $300 a dose price is a dealbreaker.
This in turn brings up an interesting economic question. Wouldn’t TerSera (marketers of the drug) make more money overall if they charged less? Best analogy I can think of is a movie theatre charging $5 for a pint of spring water that costs them no more than a dime. Wouldn’t they sell at least five times more if they charged a dollar?
July 20, 2021
Ryan RadeckiI would imagine the price point for Quzyttir was thoughtfully selected from a team of analysts putting together a value-based price – potentially incorporating, perhaps, the revenue gain from reduced ED LOS if the drug were to offer some more rapid discharge, etc.