
Emergency physicians must be skillful at performing certain high-risk, low-volume procedures. One of these procedures is emergent skull trephination, or burr hole placement, to decompress an expanding epidural hematoma. We are not aware that the procedure is taught routinely in emergency medicine residencies. It is not part of the Emergency Medicine Defined Key Index Procedure Minimums for board certification by the ACGME.1 According to the 2019 Model of the Clinical Practice of Emergency Medicine, intracranial hemorrhage is listed as a “critical” acuity. However, trephination to relieve pressure caused by epidural hemorrhage is not listed as a procedural skill integral to the practice of emergency medicine.2 While the list is not considered comprehensive, there are serious implications of the emergent presentation of this condition that would benefit from widespread training on this skill.
Explore This Issue
ACEP Now: Vol 41 – No 10 – October 2022There is a body of medical literature that suggests substantial outcome benefit when the procedure is appropriately employed. Multiple small studies and case reports indicate improved results in cases of expanding epidural hematomas with early intervention by emergency physicians.3-8
One study in 1996 assessed outcomes associated with traumatic epidural hematomas in 21 patients. The authors found that patients who underwent a drainage craniotomy in less than 70 minutes had a better recovery than those who had one at 90 minutes or more.4 A case described in ACEP Now in 2017 detailed the successful employment of emergency department trephination in a two-year-old male with an excellent outcome.5 Comparable outcomes have been found in similar studies.6-8 If left untreated, the reversal of symptoms becomes more difficult, and complications, including fatality, are positively correlated with increasing degree of uncal herniation.9
Indications
The presentation of epidural hematoma is variable. Classic symptoms that include a lucid interval between two episodes of loss of consciousness are said to occur only 20 percent of the time.10 In general, emergency department trephination should be considered in patients with clinical decline heralded by loss of consciousness—Glasgow Coma Scale less than 8—with or without ipsilateral anisocoria in the presence of a known epidural hematoma, without available neurosurgery. Whenever possible, neurosurgical consultation should take place prior to the initiation of the procedure, and a computed tomography (CT) scan should ideally be obtained prior to initiation of the intervention.11
Procedure Description
On patients, the skull thickness should be assessed utilizing CT and the drill depth set. The procedure site is typically located approximately 2 cm superior and 2 cm anterior to the tragus. A 4-cm vertical skin incision is made. A curved mosquito clamp or periosteal elevator will assist in visualizing the skull. One type of drill that has been described in the literature is the Galt trephine, which has a T handle that is used to create hand pressure coupled with a twisting motion to cut into the skull.3 Figure E shows the instrument we have used. The Galt trephine will create a circular piece of bone that can be removed either with the periosteal elevator or a mosquito clamp. The bone fragment should be placed in saline. At this point, the blood should drain spontaneously. But if needed, small tubing connected to a syringe can be inserted into the wound to aspirate blood.5,12
Creation and Use of Training Model
In order to facilitate training, we first used the Galt trephine on an embalmed cadaver model. However, we learned that it required significant effort and time to enter the epidural space on the cadaver. Additionally, we recognized that the cadaver model was limited in availability and portability as a training model for physicians. So, this led to conceptualizing the simple, inexpensive model described below. Further, we were able to utilize the model as a procedural trainer in a recent mass casualty incident training exercise at Rocky Vista University College of Osteopathic Medicine in Parker, Colorado.
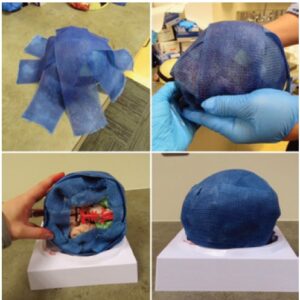
The model we created is constructed of a model brain (Figure A), an ice pack (evacuated of fluid and injected with simulation blood), cling wrap, tape, 3-inch-wide fiberglass casting material, and a repairable, simulation skin wrap.
The ice pack segments are drained of their fluid with an 18G needle and 10-cc syringe. Simulation blood, which can be commonly purchased online, is mixed with water to approximate the consistency of real blood and is then injected back into the segments using the same 18G needle and 10-cc syringe (Figure B). Each segment holds approximately 8 cc of simulation blood. The three continuous ice pack segments are then wrapped with cling wrap and secured to the brain model bilaterally in the temporal regions using strong adhesive tape (Figure A). Fiberglass casting material is molded around the model and blood packets to create a simulated skull, as seen in Figure C. After the fiberglass has dried and hardened, the repairable, simulation skin is wrapped around the skill model (Figure D).
This model results in a burr hole procedure that can be reproducibly performed. The model can provide physicians with a safe way to develop knowledge and muscle memory of a low-volume, high-risk procedure that would otherwise be typically left to didactic instruction and in-the-moment training.
We employed this model during the mass casualty incident training at Rocky Vista University College of Osteopathic Medicine. Medical students were provided a patient case presenting with an expanding epidural hematoma, as identified by signs and symptoms (initial loss of consciousness with lucid interval, followed by rapid decline in Glasgow Coma Scale and ipsilateral fixed and dilated pupil) and a provided CT scan image depicting an epidural hematoma. Once the indications for the burr hole procedure were recognized by the student, and the “patient” appropriately sedated and intubated, the model was provided to perform the burr hole (Figure E).
Future Uses and Suggestions
Care needs to be taken as to the number of layers of fiberglass used to create the skull. We found that too many or too few layers created less than realistic conditions for drilling. Three layers of fiberglass seemed to provide adequate thickness.

FIGURE D: Repairable simulated neck skin. Obtained from Strategic Operations, Inc., San Diego, CA—$52.
There are a few limitations with our model, as we originally developed it. Therefore, we suggest that this model can be modified and improved upon to provide a more realistic task trainer for the burr hole procedure. These modifications result in an even simpler construction of the model. Subsequently, we have found that using a 2-inch by 2-inch, 4-mm, resealable plastic packet filled with ten to 15 mL of artificial blood (Figure F) resulted in more predictable “blood” flow after drilling and avoided leakage as was sometimes seen from the ice-pack segments. We have also found that using a commercially available, stretchable “skull cap” to cover the fiberglass material serves as a good base for a silicone skin pad which is glued to the skullcap. The silicone skin pad can be made relatively inexpensively, or purchased, and used as an alternative to the commercial neck skin that was used initially (Figure G).
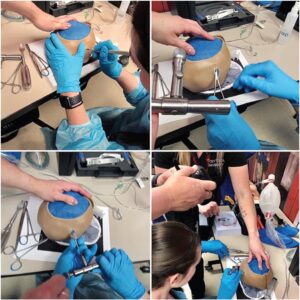
FIGURE E: Medical students simulating the burr hole procedure at Rocky Vista University College of Osteopathic Medicine, MCI training.
When implementing the use of a trephine drill in actual clinical practice, there are other options available, as the Galt trephine seems less utilized in current patient care settings. Cranial access kits can be purchased utilizing an internet search. These kits typically consist of a rotary, hand powered drill. They also provide different drill bit sizes, as well as a stopper to control depth of drilling. Cranial access kits may be used by neurosurgeons to place ventriculostomies. At the time of this writing, these kits cost approximately $1,100. Our model should be a good simulation platform for use with cranial access kits, as well as the Galt trephine drill.
Conclusion
Emergency trephination is a low-frequency, high-risk procedure. To our knowledge, there are no inexpensive portable trainers available for this procedure. Our model attempts to address this gap. We believe that with future refinements, the model may serve an important role in the training of emergency physicians and other non-neurosurgeons in the performance of this skill.
Danielle Kowal is a third-year medical student at Rocky Vista University College of Osteopathic Medicine in Parker, Colo.
Dr. Ross is an emergency physician and faculty member at Rocky Vista University College of Osteopathic Medicine in Parker, Colo. He is an associate professor and director of the rural and wilderness medicine track. He also teaches in the office of simulation in medicine and surgery (SIMS) at Rocky Vista University.
The authors have no financial conflicts of interest to disclose.
References
- Emergency medicine defined key index procedure minimums. Accreditation Council for Graduate Medical Education website. Available at https://www.acgme.org/globalassets/pfassets/programresources/em_key_index_procedure_minimums_103117.pdf. Published November 2017. Accessed September 13, 2022.
- Beeson MS, Ankel F, Bhat R, et al. The 2019 model of the clinical practice of emergency medicine. The Journal of Emergency Medicine. 2020;59(1):96-120.
- Smith SW, Clark M, Nelson J, et al. Emergency department skull trephination for epidural hematoma in patients who are awake but deteriorate rapidly. J Emerg Med. 2010;39(3):377-383.
- Cohen JE, Montero A, Israel ZH. Prognosis and clinical relevance of anisocoria-craniotomy latency for epidural hematoma in comatose patients. J Trauma. 1996;41(1):120-122.
- Beffa D. How to perform an emergency Burr Hole procedure. ACEP Now website. https://www.acepnow.com/article/perform-emergency-burr-hole-procedure/. Published December 15, 2017. Accessed September 13, 2022.
- Bulters D, Belli A. A prospective study of the time to evacuate acute subdural and extradural haematomas. Anaesthesia. 2009;64(3):277-281.
- Nelson JA. Local skull trephination before transfer is associated with favorable outcomes in cerebral herniation from epidural hematoma. Acad Emerg Med. 2011;18(1):78-85.
- Leach P, Childs C, Evans J, Johnston N, Protheroe R, King A. Transfer times for patients with extradural and subdural haematomas to neurosurgery in Greater Manchester. British Journal of Neurosurgery. 2007;21(1):11-15.
- Uzan M, Yentur E, Murat H, et al. Is it possible to recover from uncal herniation? Analysis of 71 head injured cases. Journal of neurosurgical sciences. 1998;42:89-94. PMID: 9826793.
- Price, D. Epidural hematoma management in the ED clinical presentation. Medscape website. https://emedicine.medscape.com/article/824029-clinical. Published February 2, 2022. Accessed September 13, 2022.
- O’Sullivan M, Gray W, Buckley T. Non-neurosurgical operative intervention in head-injured patients. British Journal of Neurosurgery. 1990;4:473-478.
- Wilson MH, Wise D, Davies G, et al. Emergency burr holes: “How to do it”. Scand J Trauma Resusc Emerg Med. 2012;20(24).
Pages: 1 2 3 4 | Multi-Page





No Responses to “Emergency Department Trephination (Burr Hole) for Epidural Hematoma”