
Previously called reflex sympathetic dystrophy (RSD) or causalgia, complex regional pain syndrome (CRPS) entails severe and chronic pain and disability involving a part or whole of a limb. The pain follows a non-dermatomal distribution. CRPS-1 (reflex sympathetic dystrophy) is the most common, accounting for approximately 90 percent of cases, and differs from CRPS-2 (causalgia) in that a nerve lesion typically from trauma, vascular event, or surgery is identified in the latter. Approximately 60 percent of cases involve the upper limbs, with 40 percent affecting a lower limb. CRPS typically follows a minor or moderate extremity injury, such as a wrist fracture, sprain, blunt injury, stabbing, animal bite, or elective surgery. In a minority of cases, no inciting event can be recalled or identified. The disorder typically starts within four to six weeks of the injury. Most patients with CRPS report that pain caused them to stop working.1 It may follow a stroke or myocardial infarction (“shoulder-hand syndrome”). The incidence has been reported to be as high as 28 percent following Colles’ fracture, although most cases resolved after one year. Approximately 3 to 5 percent of patients who sustain a distal radius fracture develop CRPS.2-4 Muscle weakness and changes in sweating and hair and nail growth may occur in that limb.4 Allodynia is defined as pain induced by non-painful stimuli (painful touch), whereas hyperalgesia is more pain than expected to be induced by painful stimuli. One or both should be present in making this diagnosis. Continuing pain in CRPS is disproportionate to the inciting event. Pain, sensory, trophic (hair growth increase, changes in nail growth), and motor symptoms are not confined to single nerve innervation territories.3 Vasomotor findings may include temperature asymmetry, skin color changes, sweating changes/asymmetry, or edema.5
Explore This Issue
ACEP Now: Vol 35 – No 12 – December 2016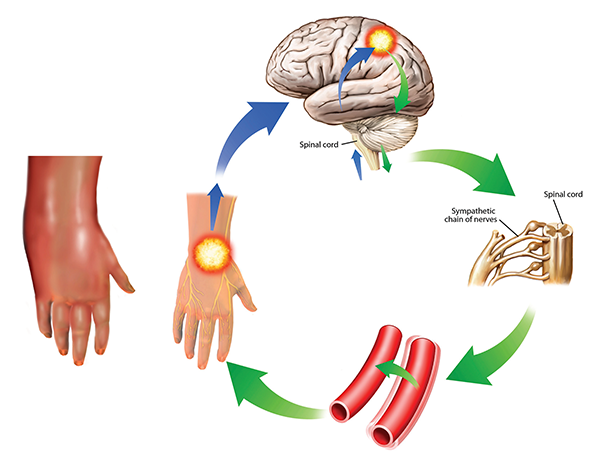
Complex regional pain syndrome
This condition is also known as reflex sympathetic dystrophy. The symptoms can worsen over time and spread through the body as they are reinforced in the cycle shown here. The sympathetic response (green) from the brain (upper right) to the pain impulses (blue) cause vessel spasms (lower right) that increase the pain. In this case, the pain and swelling are in the hand, but other areas of the body can also be affected. It is severely painful and treatment is difficult, involving drugs, electrical stimulation, and psychological and neurological forms of therapy.
John T. Alesi / Science Source
There may be objective findings in the patient with CRPS—for example, side-to-side difference in skin temperature, or osteopenia and osteoporosis with abnormalities on bone scan/scintigraphy or on plain X-rays with both hands on one film. Other potential testing may include MRI, which may demonstrate bone marrow edema and autonomic testing of sweat output. The diagnosis of CRPS is excluded by the existence of another condition that would better explain the degree of pain and dysfunction.6 Women are affected more often than men. It is most frequent in those ages 61 to 70.7 Approximately 20 percent of patients are able to resume previous activities.8
The diagnosis of CRPS has historically been one of exclusion. The Budapest criteria were established in 2003 to objectively diagnose CRPS and have been shown to have a sensitivity of 99 percent and a specificity of 79 percent.6 These four criteria must be met for the diagnosis: 1) pain out of proportion to the inciting injury; 2) patient’s reported history of at least one symptom in three of the four categories of sensory (eg, hyperesthesia, or increased pain response to a mildly painful stimulus, and allodynia, or a painful response to a nonpainful stimuli), vasomotor changes (eg, temperature or skin color asymmetry), sudomotor/edema changes (eg, asymmetric limb edema or sweating), and motor/trophic changes (eg, decreased range of motion; weakness or atrophy; tremor; or hair, nail, or skin changes); 3) the clinician’s own observation of at least two symptoms that fall into two of the previously mentioned categories; and 4) no other explanation for the patient’s symptoms (see Table 1).
Treatment
Several treatment options are available. There is evidence for posttraumatic inflammation in CRPS, so nonsteroidal anti-inflammatory drug or steroid therapy in the acute stage is reasonable. A high dose of prednisolone has been proposed as therapy.6 Additional oral medications that can be used include tricyclic antidepressants and anticonvulsants such as gabapentin and carbamazepine. There is some evidence that gabapentin or carbamazepine therapy may reduce pain in CRPS.6 Bisphosphonates inhibit the activity of osteoclasts. Pamidronate 60 mg as one intravenous dose has been proposed.9,10
Topical dimethyl sulfoxide (DMSO 50 percent cream) has been shown to have a positive effect on pain, presumably through its role as a free-radical scavenger. Applied for two months, it may provide significant pain relief.10 Other topical medications such as capsaicin, lidocaine 5 percent, or eutectic mixture of local anesthetics (EMLA) cream may be used as well. Patients should be encouraged to use the affected extremity. Specifically, splints, slings, and immobilizing devices, particularly for prolonged periods, should be avoided.9
Intravenous immune globulin has been used effectively, with the rationale that CRPS is related to the presence of unidentified neural antibodies. However, use in the emergency setting has not been recommended.11 The role of opioids as a second- or third-line option for therapy (“rescue dosing”) has not been completely defined. Opioids have not been recommended, except with specific advice and input from a pain management specialist.9 There are concerns for tolerance, cognitive impairment, and opioid-induced hyperalgesia.6 Non-pharmacologic treatment options include appropriate physical and occupational therapy regimens such as isotonic strengthening, passive gentle range of motion, aerobic conditioning, aquatic therapy, and ergonomics.
Vitamin C is proposed to prevent CRPS. However, there is not enough evidence to recommend this for routine therapy following, for example, a Colles’ fracture. More invasive therapies exist but are outside the scope of emergency medicine. Spinal cord stimulation and intrathecal baclofen pumps have led to a decline in pain. Stellate ganglion block, brachial plexus block, and lumbar sympathetic block have all been used with some success.6
The role of lidocaine or ketamine in the management of chronic painful conditions is evolving. Topical lidocaine comes in patches, creams, and ointments. These have been used in CRPS. Options for CRPS include EMLA and 5 percent lidocaine-impregnated patch.12 Intravenous lidocaine has been evaluated in patients with renal colic and for acute low back pain. It has been proven effective in resolution of pain when used in doses ranging from 1.5 mg/kg to 100 mg IV. Side effects of nausea and dizziness tend to be mild and transient. One study evaluated the use of IV lidocaine infusion in CRPS patients. There was evidence for a decrease in pain response to cold stimuli and, at 3 mcg/mL infusion, a decrease in pain level.13 Lidocaine infusions have been advocated for severe and neuropathic pain, as in cancer or diabetic neuropathy. Dosage for testing purposes has been proposed at 1–3 mg/kg (100 mg being a frequently used dose) over 20 to 30 minutes. If the challenge dose is effective, then an infusion at 0.2–2 mg/kg per hour can be given, often with dramatic relief of pain.14 At this time, the role of intravenous lidocaine in the management of neuropathic pain remains to be defined.
Ketamine has been demonstrated to be efficacious in the management of pain in CRPS.15 It prevents or attenuates the hyperalgesia and allodynia of CRPS.16 As noted below, it may prove to be effective in inducing long-term pain relief when administered for four to 14 days.17
Intravenous ketamine in subdissociative doses may be effective in CRPS. A recent study of low-dose ketamine when used as an infusion initially of approximately 5 mg/hour titrated up to as much as 30 mg/hour for a 70 kg patient over 4.2 days in 60 patients with CRPS demonstrated significant pain relief during a 12-week study period, not simply during its administration.18 The chance for clinically significant pain reduction for 11 weeks after therapy must be weighed against the cost of hospitalization.
Dr. Glauser is on the faculty of the emergency medicine residency program at MetroHealth Medical Center and professor of emergency medicine at Case Western Reserve University, both in Cleveland. Dr. Money is assistant clinical professor at the Spine Institute at the University of Michigan in Ann Arbor.
References
- Bickerstaff DR, Kania JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol. 1994;33:240-248.
- Zyluk A. The natural history of post-traumatic reflex sympathetic dystrophy. J Hand Surg Br. 1998;23:20-23.
- Birklein F, O’Neill DO, Schlereth T. Complex regional pain syndrome: an optimistic perspective. Neurology. 2015;84:89-96.
- Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637-648.
- Harden RN, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest criteria”) for complex regional pain syndrome. Pain. 2010;150:268-274.
- Harden RN, Oaklander AL, Burton AW, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14:180-229.
- de Mos M, de Bruijin AG, Huygen FJ, et al. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12-20.
- Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012-1016.
- Turner-Stokes L, Goebel A, Guideline Development Group. Complex regional pain syndrome in adults: concise guidance. Clin Med (Lond). 2011;11(6):596-600.
- Bussa M, Guttilla D, Lucia M, et al. Complex regional pain syndrome type I: a comprehensive review. Acta Anaesthesiologi Scand. 2015;59:685-697.
- Goebel A, Baranowski A, Maurer K, et al. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152:152-158.
- D’Arcy Y. Targeted topical analgesics for acute pain. Pain Med News. 2014;12(12):56-63.
- Wallace MS, Ridgeway BM, Leung AY, et al. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000;92:75-83.
- Ferrini R, Paice JA. How to initiate and monitor infusional lidocaine for severe and/or neuropathic pain. J Support Oncol. 2004;2:90-94.
- Schwartzman RJ, Alexander GM, Grothusen JR. The use of ketamine in complex regional pain syndrome: possible mechanisms. Expert Rev Neurother. 2011;11:719-734.
- Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60:341-348.
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77(2):357-367.
- Sigtermans MJ, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145:304-311.
Pages: 1 2 3 4 | Multi-Page





No Responses to “What Emergency Physicians Need to Know About Complex Regional Pain Syndrome”