The clinical exam is inherently subjective from one provider to another, but it has even been shown that individual physicians are not consistent with their own assessment of wheezing on auscultation.1 Peak expiratory flow rate, or peak flow, is another modality that has been extensively used in EDs to quantify the extent of a patient’s asthma exacerbation and response to treatment. The downside to peak flow is that it is highly dependent on the patient’s ability to properly perform the test and to consistently reproduce the test. Some investigators have explored the use of end-tidal capnography as a means of monitoring a patient’s response to treatment because it eliminates the patient cooperation variable to obtain an accurate assessment.

Figure 1. Normal, healthy capnogram.
Capnometers were initially used in submarines in World War II but now are found in nearly every ED in the country.2 A capnogram in a normal, healthy adult consists of a few separate phases (see Figure 1). Phase I occurs during the beginning of exhalation where the majority of the air is dead space, and hence, very little carbon dioxide is released, resulting in a relatively flat portion of the curve. As the patient continues to exhale, the mixed air has an increased carbon dioxide concentration, and subsequently, the capnography curve begins a steep incline; this is called Phase II. The plateau Phase (Phase III) has a decreased slope, with only a small increase in CO2 concentration, and peaks at the end-tidal point. This part of the capnogram represents the expiration of alveolar air and remains nearly horizontal because of the homogeneity of alveolar ventilation within healthy lungs.3 Once the patient begins inhalation (Phase 0), the end-tidal drops precipitously and ends the waveform. In a healthy patient, the angle formed (α) from Phase II to Phase III is approximately 110 degrees, and the angle formed between Phase III and 0 (β) is typically about 90 degrees.4
When asthmatics present with a severe attack, their waveform changes in such a manner that it begins to resemble a shark fin. The bronchoconstriction of the small airways during an asthma exacerbation causes a decrease in alveolar ventilation in different regions of the lung.
When asthmatics present with a severe attack, their waveform changes in such a manner
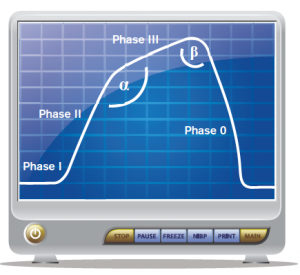
Figure 2. Bronchospasm on capnogram.
that it begins to resemble a shark fin (see Figure 2). The bronchoconstriction of the small airways during an asthma exacerbation causes a decrease in alveolar ventilation in different regions of the lung. Each portion of the lung is associated with its own ventilation-perfusion ratio (V:Q) that subsequently determines its respective PaCO2. During expiration in an asthma exacerbation, the areas of the lung with less bronchoconstriction have a lower PaCO2 and will preferentially be expired first. On the other hand, the regions of the lung with a greater degree of obstruction will have a higher PaCO2 and will have delayed emptying. Some authors refer to these differences in expiration of CO2 during an asthma exacerbation as desynchronization.3,5 The desynchronization of alveolar emptying causes changes within the capnogram waveform; the slope of Phase II decreases, the slope of Phase III increases as the more highly obstructed alveoli expire their retained CO2 in a delayed fashion, and these changes in the slope result in an increased α angle (see Figure 2).3 The focus of using a capnogram as an assessment tool in asthmatics is not on the numeric end-tidal value but more so to evaluate the changes in the waveform morphology.
One of the larger investigations into capnographic waveforms and peak flow meter measurement was in 2009.6 One hundred patients with acute asthma exacerbations were enrolled in the study. Prior to receiving any therapy, a peak flow measurement was taken and a capnogram was recorded (nasal cannula sampling capnometry). Once the attending physician felt comfortable that the patient was “fit for discharge,” another set of these parameters was ascertained. The investigators did not find a significant (P=0.35) change in the slope of Phase II of the capnogram between pre- and post-treatment. However, they were able to find a significant (P<0.001) decrease in the slope of Phase III and a significant (P<0.001) decrease in the α angle.6 Despite these noteworthy changes in the Phase III slope and α angle, there was poor correlation between these indices and the changes in peak flow pre- and post-treatment. Yaron et al made it a little bit easier by looking only at the slope of Phase III and its relation to bronchospasm in the ED.7 Similar to the previous study, they found a significant decrease in the slope of Phase III from 0.27 ± 0.05 to 0.19 ± 0.07 (P<0.005) between pre- and post-treatment with 2.5 mg of albuterol. Unlike the previously discussed study from Nik Hisamuddin et al, they were able to show a correlation (r=0.84) between the slope of Phase III to the log of the predicted peak flow.7 How does this hodgepodge of numbers actually pertain to clinical medicine? Let’s use a clinical case with capnograms and apply these ideas to help decide on the most appropriate disposition of the asthmatic patient.
The Case
A 45-year-old female with an extensive history of asthma presents to the ED with the complaint of having an asthma exacerbation. Her respiratory rate is 24, and she is mildly hypoxic (91 percent) on room air and has moderate inspiratory and expiratory wheezing that is scattered among all lung fields along with subcostal accessory muscle use. Before the respiratory therapist gets an albuterol/ipratropium bromide treatment going, you attempt to get a peak flow. The patient blows into the peak flow only to get 125 L/min, but
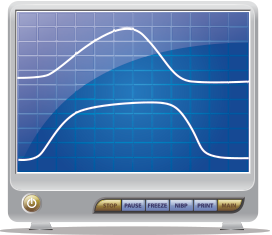
Figure 3. Top capnogram is before any intervention, and bottom is after treatment for the patient’s asthma.
you notice she isn’t using it correctly. You choose not to delay treatment any further and quickly obtain a baseline capnogram prior to the treatment. Figure 3 (top) shows her initial end-tidal capnogram. Subsequently, Figure 3 is after 10 mg of albuterol, 0.5 mg of ipratropium bromide, and 60 mg of prednisone. Despite the fact that her respiratory rate normalized, her wheezing nearly resolved, and her overall clinical status vastly improved, when you obtain a peak flow post-treatment, her best is only 150 L/min. Does she need to be admitted because of the peak flow measurements? You instead obtain a post-treatment capnogram and compare it to the pre-treatment result. Even with the naked eye, you can see the improvement in her end-tidal capnogram going from a shark fin appearance pre-treatment to a more normal waveform post-treatment. Taking into account the entire clinical picture and capnogram, the patient is discharged home.
The focus of using a capnogram as an assessment tool in asthmatics is not on the numeric end-tidal value but more so to evaluate the changes in the waveform morphology.
Using end-tidal capnography to monitor asthma exacerbations is not something new. You et al did one of the first investigations into capnography’s utility in asthma in 1992.8 Since then, many of the other studies have been making attempts to correlate these end-tidal indices with peak flow measurements. Peak flow measurements are largely dependent on the diameter of larger airways, whereas the capnogram is more dependent on the smaller airways; this may make it difficult to correlate the two values.6,7 With the improved accessibility of end-tidal monitors in nearly all EDs, why not hook your next asthmatic patient up to the monitor, take a look at the waveform, or even print it out prior to giving any treatments? Although more studies need to be performed and the results validated before this becomes the standard of care for asthmatics, the days of huffin’ and puffin’ into peak flows may be a thing of the past.
 Dr. McGovern is an emergency medicine resident at St. Joseph’s Regional Medical Center in Paterson, New Jersey.
Dr. McGovern is an emergency medicine resident at St. Joseph’s Regional Medical Center in Paterson, New Jersey.
 Dr. Justin McNamee is an attending physician at Emergency Medicine Professionals in Ormond Beach, Florida.
Dr. Justin McNamee is an attending physician at Emergency Medicine Professionals in Ormond Beach, Florida.
References
- Pasterkamp H, Wiebicke W, Fenton R. Subjective assessment vs computer analysis of wheezing in asthma. Chest. 1987;91:376-381.
- O’Flaherty D. Capnography: principles and practice. London: BMJ Publishing Group; 1994.
- You B, Peslin R, Duvivier C, et al. Expiratory capnography in asthma: evaluation of various shape indices. Eur Respir J. 1994;7:318-323.
- Rasera CC, Gewehr PM. Association between capnogram and respiratory flow rate waveforms during invasive mechanical ventilation. Int J Biosci Bioche Bioinforma. 2013;3:80-84.
- Howe TA, Jaalam K, Ahmad R, et al. The use of end-tidal capnography to monitor non-intubated patients presenting with acute exacerbation of asthma in the emergency department. J Emerg Med. 2011;41:581-589.
- Nik Hisamuddin NAR, Rashidi A, Chew KS, et al. Correlations between capnographic waveforms and peak flow meter measurement in the emergency department management of asthma. Int J Emerg Med. 2009;2:83-89.
- Yaron M, Padyk P, Hutsinpiller M, et al. Utility of the expiratory capnogram in the assessment of bronchospasm. Ann Emerg Med. 1996;28:403-407.
- You B, Mayeux D, Rkiek B, et al. Expiratory capnography in asthma. Perspectives in the use and monitoring in children. Rev Mal Respir. 1992;9:547-552.
Pages: 1 2 3 | Multi-Page




No Responses to “How to Use End-tidal Capnography to Monitor Asthmatic Patients”