
Editors’ Note: This article was accepted on Sept. 7, 2021, and was accurate at that time. Because information about COVID-19 is evolving rapidly, please verify these recommendations and information.
Explore This Issue
ACEP Now: Vol 40 – No 11 – November 2021The COVID-19 pandemic continues to affect billions throughout the world. The rapidly growing evidence base creates a challenge for clinicians worldwide. The dynamic nature of this evidence further makes it difficult to create timely clinical guidelines, which are normally created on the scale of months to years. Thus, professional societies have adjusted their methods on how to develop guidelines during the pandemic.
The ACEP Clinical Policies Committee regularly assesses the guidelines produced by other medical specialty societies facilitated by a presentation and discussion led by the Emergency Medicine Residents’ Association (EMRA) representative to the Clinical Policies Committee. In light of the pandemic, the Surviving Sepsis Campaign created a dedicated panel, the Surviving Sepsis Campaign (SCC) COVID-19 panel, to establish and maintain guidelines to address the ever-growing body of evidence. Here we will briefly examine the “Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU.”
Guideline Process
The SSC implemented a living guideline model to provide continually updated guidance on the treatment of COVID-19. The panel released its original guideline on the management of COVID-19 in June 2020 with a subsequent update published in March 2021.1,2 The SSC COVID-19 panel includes a diverse range of experts from guideline development, infection control, infectious diseases and microbiology, critical care, hematology and thrombosis, surgery, emergency medicine, nursing, pharmacy, and public health. Eight new members were added to the panel from the prior iteration. All members had to disclose their conflict of interests (COI) and were not able to vote if they had a COI related to the guideline question. The panel utilized the GRADE methodology and the Evidence to Decision (EtD) Framework to develop the recommendations.3,4 The EtD Framework provides a structured approach that helps make the assessment and integration of the evidence, and other patient-centered considerations more systematic and explicit to generate rigorous recommendations.
Professional medical librarians performed a literature search through Cochrane Central Register of Controlled Trials and the National Library of Medicine’s MEDLINE databases. Trained reviewers screened the literature search and removed duplicates. Random-effects meta-analysis was done when applicable, based upon the data. The GRADE approach was utilized to assess the quality of evidence.3 Only direct evidence (evidence generated from studies on COVID-19) was included in the March 2021 update, as opposed to the prior guideline which included indirect evidence (evidence from more general disease process such as acute respiratory distress syndrome). The exception to this is questions regarding anticoagulation, as direct evidence was not available.
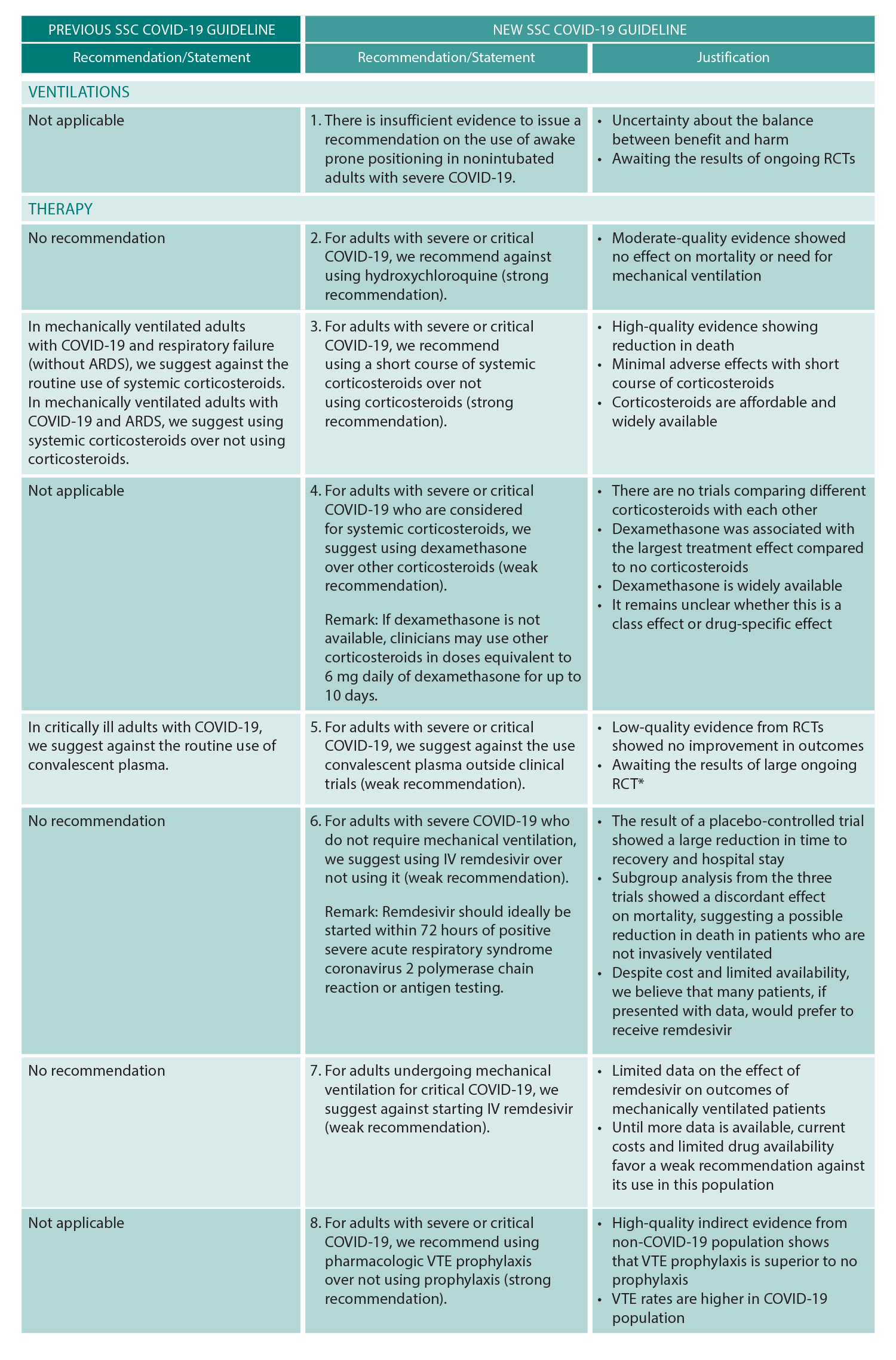
(click for larger image) Table 1: SSC COVID-19 Guidelines
ARDS = acute respiratory distress syndrome, RCT = randomized controlled trial, VTE = venous thromboembolism.
Reprinted with permission from Crit Care Med. 2021;49(3):e219-e234.
* Editor’s note: The data regarding COVID changes rapidly. A large RCT, the C3PO trial, showed negative effects of convalescent plasma in August 2021.
The guideline utilizes “we recommend” for strong recommendations and “we suggest” for weak recommendations. Ultimately three new recommendations and six updated recommendations were added to the prior SSC COVID-19 guidelines. New and updated recommendations will be forthcoming as the COVID-19 evidence base grows in accordance with the above stated living guideline methodology.
Briefly, the guideline recommends against the use of hydroxychloroquine and therapeutic anticoagulation; suggests against convalescent plasma; recommends the use of pharmacologic venous thromboembolism prophylaxis and corticosteroids, suggesting dexamethasone as the corticosteroid choice; and suggests remdesivir in severe COVID-19 patients not needing mechanical ventilation. See Table 1 for a summary of the guideline updates.
The guideline uses the WHO definition of severe covid which, in adults, includes clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 <90 percent on room air.5 Plus-circle
Dr. Hickey is EMRA Representative to the Clinical Policies Committee 2019–2021. Dr. Villars is EMRA Representative to the Clinical Policies Committee 2021–2022.
References
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887.
- Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign: guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219-e234.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
- Moberg J, Oxman AD, Rosenbaum S, et al. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45.
- World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020 (No. WHO/2019-nCoV/clinical/2020.5). New York, NY: World Health Organization; 2020.
- Korley FK, Durkalski-Mauldin V, Yeatts SD, et al; SIREN-C3PO Investigators. Early convalescent plasma for high-risk outpatients with Covid-19 [online ahead of print Aug 18]. N Engl J Med. 2021.
Pages: 1 2 | Multi-Page



No Responses to “The Surviving Sepsis Campaign Creates COVID-19 Guideline”