
The diagnosis and treatment of high-altitude illness (HAI) require an understanding of the interplay between physics and physiology. As altitude increases, pressure decreases, affecting the partial pressure of oxygen and thus decreasing the amount of oxygen diffusing into the tissues. This hypobaric hypoxia results in a cascade of events known as HAI. In an effort to acclimate, the respiratory rate increases, leading to respiratory alkalosis with metabolic compensation. This also causes an overall left shift of the oxygen-hemoglobin dissociation curve, increasing oxygen uptake in the lungs. Hypoxemia causes increased release of erythropoietin, leading to an increase in red blood cell production and overall better oxygen-carrying capacity to the tissues.
Explore This Issue
ACEP Now: Vol 38 – No 05 – May 2019The most important syndromes that make up the spectrum of HAI are high-altitude pulmonary edema (HAPE) and acute mountain sickness (AMS), which can progress to high-altitude cerebral edema (HACE). Younger athletes and males are at greater risk of HAI since they are more likely to engage in vigorous activity prior to acclimatization or continue ascent despite symptoms. Other risk factors include chronic obstructive pulmonary disease, restrictive lung disease, cystic fibrosis, pulmonary hypertension, congestive heart failure, and sickle cell disease. Contrary to popular belief, neither well-controlled asthma nor pregnancy (up to 3,000 meters) increase the risk of HAI.
High-Altitude Pulmonary Edema
There are two types of HAPE: classic, which occurs in low-altitude residents who rapidly ascend, and reentry, which occurs in high-altitude residents re-ascending after being at low altitudes. The pathophysiology of HAPE consists of breakdown of the pulmonary blood-gas barrier secondary to increased pulmonary artery pressure and uneven pulmonary vasoconstriction resulting in fluid accumulation within the alveoli. This typically occurs around 3,000 meters. Patients present with a dry cough that progresses to a productive cough with frothy pink sputum and increased dyspnea within four to six days of arrival at altitude. Patients demonstrate tachycardia, tachypnea, inspiratory crackles, and low pulse oximetry on physical exam. Chest X-ray reveals patchy infiltrates, but it is not required to make a diagnosis.1
The treatment of stable patients with HAPE involves simply giving oxygen via high-flow nasal cannula and decreasing cold exposure to resolve the elevation in pulmonary artery pressure. Unstable patients should descend as soon as possible, and if that is not possible, use hyperbaric therapy as indicated.1 Medications can be used for treatment. However, they are more effective as preventative measures. Nifedipine can reduce pulmonary vascular resistance and decrease pulmonary artery pressure, and phosphodiesterase 5 inhibitors can increase cyclic guanosine 3’,5’-monophosphate (cGMP) to augment the pulmonary vasodilatory effects of nitric oxide. Nitric oxide is a potent pulmonary vasodilator, released from endothelial cells, that decreases hypoxic pulmonary vasoconstriction and the pulmonary hypertension associated with HAPE. Inhaled beta-agonists can be used as an adjunct, but they have limited effectiveness as a sole treatment option.2–4
Acute Mountain Sickness and High-Altitude Cerebral Edema
The pathophysiologies of the neurological forms of HAI—AMS and HACE—are similar in that there is an increase in the permeability of the blood-brain barrier causing reversible vasogenic edema. The mechanism of this increased permeability is unclear. There is a possible increase in cerebral blood flow, loss of intracranial pressure autoregulation, and resultant alterations in endothelial permeability via increased nitric oxide levels and increased vascular endothelial growth factor (VEGF), which promotes angiogenesis. AMS and HACE are along a spectrum of disease, with AMS occurring around 1,500 meters with typical transition to HACE at more than 4,000 meters. The diagnosis for AMS is clinical, with symptoms that resemble a hangover, such as headache, anorexia, nausea, and vomiting.1 Onset of AMS generally occurs within six to 12 hours of reaching altitude and resolves within one day, but it can recur as ascent continues. The Lake Louise AMS score is the gold standard to self-monitor for AMS during ascent or for a clinician evaluating a patient.5,6 HACE is a clinical diagnosis, with onset at 12 hours to three days from ascent. The patient will present with ataxia, encephalopathy, and a progressive decline of mental function and level of consciousness. Patients may first only appear to be withdrawn; clinical suspicion should be high. Physical examination will reveal a patient with impaired finger-to-nose or heel-to-shin testing. All labs and imaging are fairly nonspecific, but they may show leukocytosis and possibly cerebral edema.1,7
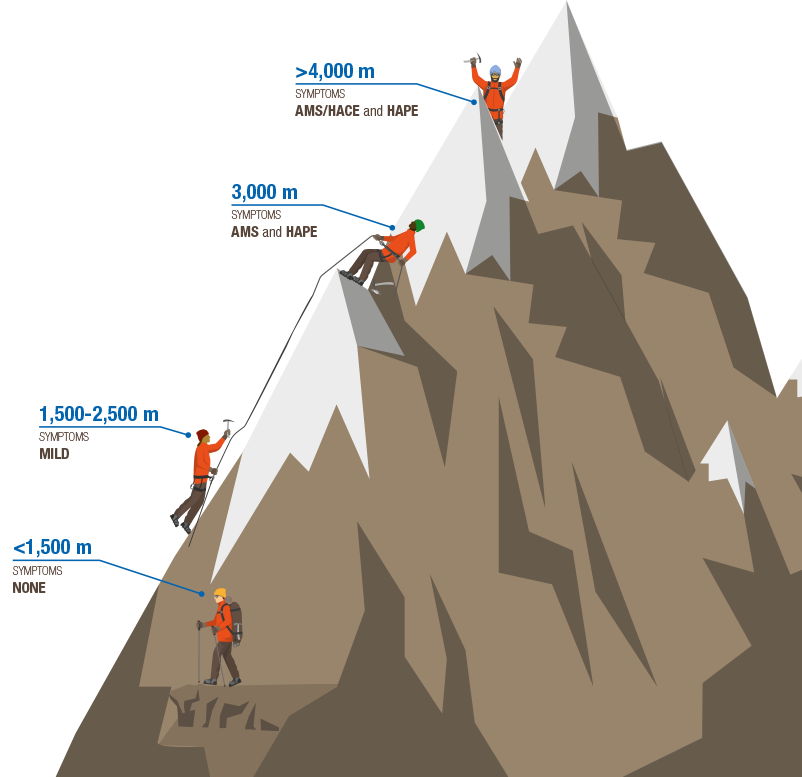
TYpical Elevations for High-Altitude Illness
ILLUSTRATION: Chris Whissen & shutterstock.com
Treatment of AMS consists of symptomatic management with nonsteroidal anti-inflammatory drugs (NSIADs), antiemetics, and a pause in ascent for 48 hours for acclimatization to occur. Treatment for HACE is immediate descent. If that is not possible, the patient should be treated in a hyperbaric chamber, along with 2–4 L nasal cannula and dexamethasone for alleviation of symptoms related to cerebral edema. In the event the patient becomes unresponsive, consider protecting the airway.2,4
Prescription medications can be used for treatment but are better as preventative measures. Acetazolamide, a carbonic anhydrase inhibitor, initiates metabolic acidosis, theoretically stimulating respiratory drive and hastening acclimatization, but its side effects can be mildly irritating, with peripheral paresthesia, polyuria, and a metallic aftertaste. Dexamethasone can be used to alleviate symptoms, but it does not accelerate acclimatization. The risk with dexamethasone is that it masks symptoms and therefore may increase the risk of AMS progressing to HACE as ascent continues.2,4
For AMS and HACE, the gold standard for prevention is gradual ascent, acetazolamide, and +/- dexamethasone. Dexamethasone is typically used in prevention of AMS/HACE for patients who require rapid ascent but does not hasten acclimatization. There are multiple drugs that, when compared to placebo, do show improvement for prevention, such as acetazolamide and dexamethasone. As a selective 5-hydroxytryptamine receptor agonist and a cerebral vasoconstrictor, sumatriptan shows promising results for AMS prevention; however, only one study has been published, which showed decreased prevalence of AMS in sumatriptan versus placebo when used for prophylaxis.8 Further studies would need to be completed prior to utilizing sumatriptan as a preventative medication. Antioxidants, magnesium, ginkgo biloba, and cocoa leave use have very limited data and scattered anecdotal reports, with no significant difference shown when compared to acetazolamide and dexamethasone.2,4,9
Pediatric Patients
Pediatric patients present a unique cohort in risk factors and presentation for HAI. Risk factors for HAI include congenital cardiopulmonary disease (eg, cardiac shunting, pulmonary hypertension), cystic fibrosis, sickle cell disease, and Down’s syndrome. Also at risk are any infants born preterm, those less than six weeks old, and those who required oxygen within their first year of life.
Children typically experience reentry HAPE, the risk of which is increased with any respiratory infection. Kids have increased respiratory distress over one to two days that presents as decreased playfulness, disrupted sleep, and increased fussiness and crying. Treatment and prevention are the same as HAPE in adults.
In AMS and HACE, younger kids present with decreased playfulness, poor sleep, and increased fussiness. Teenagers present similar to adults with headache, shortness of breath, nausea, vomiting, and anorexia. The diagnosis is clinical and treatment is the same as for adults. Dexamethasone should not be used for prevention in children as it can lead to adrenocortical suppression.1,10
 Dr. Jacobson is an emergency medicine resident physician at Mayo Clinic in Rochester, Minnesota.
Dr. Jacobson is an emergency medicine resident physician at Mayo Clinic in Rochester, Minnesota.
 Dr. Raukar is an emergency medicine consultant and associate professor at Mayo Clinic in Rochester, Minnesota.
Dr. Raukar is an emergency medicine consultant and associate professor at Mayo Clinic in Rochester, Minnesota.
References
- Auerbach P, Cushing T, Harris N. Auerbach’s Wilderness Medicine. 7th ed. Philadelphia: Elsevier; 2017:1-39.
- Luks AM, McIntosh SE, Grissom CK, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014;25(4 suppl):S4-S14.
- Nieto Estrada VH, Molano Franco D, Medina RD, et al. Interventions for preventing high altitude illness: Part 1. Commonly‐used classes of drugs. Cochrane Database Syst Rev. 2017;6:CD009761.
- Simancas‐Racines D, Arevalo‐Rodriguez I, Osorio D, et al. Interventions for treating acute high altitude illness. Cochrane Database Syst Rev. 2018;6:CD009567.
- Roach RC, Hackett PH, Oelz O, et al. The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol. 2018;19(1):4-6.
- Meier D, Collet TH, Locatelli I, et al. Does this patient have acute mountain sickness? The rational clinical examination systematic review. JAMA. 2017;318(18):1810-1819.
- Gallagher S, Hackett P. Acute mountain sickness and high altitude cerebral edema. UpToDate. 2018. Accessed April 22, 2019.
- Jafarian S, Gorouhi F, Salimi S, et al. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neuro. 2007;62(3):273-277.
- Seupaul RA, Welch JL, Malka ST, et al. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Ann Emerg Med. 2012;59(4):307-317.e301.
- Pollard AJ, Niermeyer S, Barry P, et al. Children at high altitude: an international consensus statement by an ad hoc committee of the International Society for Mountain Medicine, March 12, 2001. High Alt Med Biol. 2001;2(3):389-403.
Pages: 1 2 3 4 | Multi-Page


One Response to “Tips for Spotting and Treating High-Altitude Illness”
June 9, 2019
Timothy Peterson MDI love your succinct synopsis, as a physician who has practiced at 9,300 feet for thirty-one years.
Comments:
1.) We prefer O2, acetaminophen and ondansetron to NSAIDS in the nausea scenario. NSAIDS recommendations persist based on poorly designed earlier NSAID prevention studies which have been discounted. Why give NSAIDS to folks with nausea and vomiting?
2.) For those of us in the real world, the Lake Louise score is academic and impractical from the perspective of having treated over five thousand AMS patients. We take a history, do an exam, look for clinical improvement in patient w/o concurrent medical illness after 20 min 02 trial, then make a treatment decision. A score sounds good but does not help.