We know the ongoing shortage of lifesaving medicines is one of the biggest problems emergency physicians deal with on a daily basis. You shouldn’t have to wonder what medicines might be available on any given day. You shouldn’t have to constantly find alternatives for drugs that aren’t available, and your time shouldn’t be spent being trained and retrained about what drugs to use and what new protocols are in place every time a new drug shortage is announced.
Explore This Issue
ACEP Now: Vol 38 – No 05 – May 2019Not only do drug shortages make it harder for emergency physicians to practice, but this problem jeopardizes the health of ED patients. The medication substitutes are often less effective or come with different side effect profiles.
ACEP has been a leading voice on this issue, bringing it to public attention and pushing for progress at the federal level. Below is a timeline of our federal advocacy activities related to drug shortages:
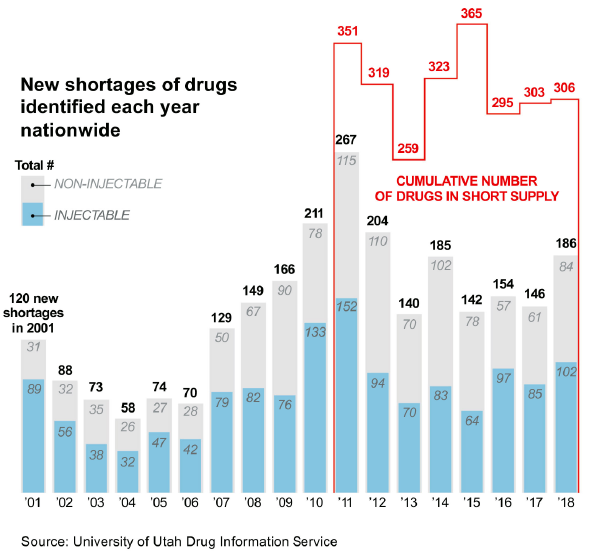
May 2018
- ACEP conducted a survey of members and found that nine out of 10 emergency physicians had experienced a drug shortage in the last month.
- We drafted a congressional sign-on letter urging US Food and Drug Administration (FDA) Commissioner Scott Gottlieb to establish a task force to identify the root causes of drug shortages and develop recommendations to address them. We then secured bipartisan, bicameral sponsors for the House and Senate letters from Rep. Brett Guthrie (R-KY), Rep. Mike Doyle (D-PA), Sen. Bill Cassidy, MD (R-LA), and Sen. Chris Murphy (D-CT), respectively.
- ACEP’s public relations team distributed a press release of drug shortage survey results in the lead up to the 2018 Leadership & Advocacy Conference (LAC).
- At LAC on May 22, 2018, the drug shortage problem was one of two topics we raised on the Hill (the other being our opioid bills). LAC participants urged members of Congress to sign the letter asking the FDA to establish the task force. Ultimately, the House letter closed with 107 representative signatures, and the Senate letter was signed by 31 senators.
June 2018
- The New York Times reached out to ACEP after seeing our press release on the drug shortage survey results, and we worked with them to develop an article that brought more national attention to the issue.1
“So many substances are short, and we’re dancing every shift,” said Dr. James Augustine, an [emergency physician] in Cincinnati.
— The New York Times, July 1, 2018
July 2018
- In direct response to the congressional letters authored by ACEP, FDA Commissioner Gottlieb announced the creation of a new Drug Shortage Task Force charged with identifying and addressing the root causes of drug shortages affecting the health care system. This was an important step forward for ACEP’s drug shortage advocacy efforts; it was exactly what our congressional letter had requested.
September 2018
- ACEP Board member Aisha Liferidge, MD, MPH, FACEP, participated in a two-day workshop hosted by the National Academies of Sciences, Engineering, and Medicine (NASEM) on medical product shortages during disasters. Dr. Liferidge discussed the unique challenges emergency physicians and their patients face during natural disasters and disease outbreaks and further discussed opportunities to lessen the effects of medical product shortages through information sharing; improved supply-chain infrastructure; and enhanced collaboration among public, private, and nonprofit stakeholders.
- Then-ACEP President Paul Kivela, MD, MBA, FACEP, attended a drug shortage summit hosted by the American Society of Anesthesiologists, American Hospital Association, and American Society of Health-System Pharmacists. The summit focused on the national security implications of drug shortages and ways to improve the nation’s health care infrastructure. Dr. Kivela engaged government speakers about steps that could be taken to lessen shortages for essential medications needed on a daily basis in the emergency department.
October 2018
- ACEP President Vidor Friedman, MD, FACEP, participated in a listening session with the FDA Drug Shortage Task Force. ACEP was one of only 10 groups invited to participate, and Dr. Friedman provided important perspectives on how drug shortages impact care for emergency patients.
November 2018
- ACEP attended an FDA Drug Shortage Task Force public meeting.
January 2019
- ACEP submitted its official response to the FDA Drug Shortage Task Force. We explained how emergency physicians are directly affected by drug shortages each day and how much it negatively affects patient care.
“Shortages of commonly used but essential medications remain an acute problem throughout the health care system, but these shortages tend to disproportionately affect emergency medicine (both hospital and pre-hospital) due to its reliance upon generic medications for rapid sequence intubation, seizures, antidotes, resuscitation, as well as analgesics, antiemetics, and anticoagulants.”
— ACEP’s letter of response to the FDA Drug Shortage Task Force
May 2019
- ACEP is waiting for the FDA Drug Shortage Task Force to submit its findings and final report to Congress. Based on its recommendations, we expect the House of Representatives and Senate to develop legislation to address the challenges and problems identified in the report. ACEP’s Washington, D.C., staff will be actively involved with those discussions and the drafting of potential legislative solutions. Stay tuned. When the time comes, we will be asking for your help to communicate ACEP’s recommended solutions to lawmakers.
Stay Updated
ACEP is dedicated to providing emergency physicians a strong and unified voice in Washington, D.C., speaking out on the issues that matter most to you and your patients. Want to stay apprised of ACEP’s ongoing federal legislative activities? Sign up for the 911 Legislative Network, the premier grassroots network for emergency physicians. Find continual updates about all of ACEP’s advocacy work, drug shortages and beyond, on the Federal Advocacy page.
Ms. Grantham is a communications manager at ACEP.
Reference
- Thomas K. Emergency rooms run out of vital drugs, and patients are feeling it. New York Times. July 1, 2018. Accessed April 10, 2019.
Pages: 1 2 | Multi-Page





No Responses to “Update on ACEP’s Drug Shortage Advocacy Efforts”