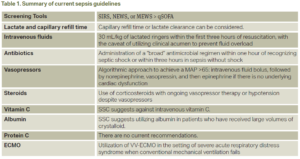
Sepsis is a life-threatening organ dysfunction secondary to a dysregulated host response to an infection; an estimated 48.9 million cases are recorded, and 11.0 million sepsis-related deaths were reported from 1990-2017.1 From its first definition in 1991, many landmark trials have advanced the care of sepsis and septic shock. In 2001, Rivers and colleagues found that early intravenous fluid administration may improve overall in-hospital mortality rates.2 Similarly, in 2004, Kumar and colleagues demonstrated that each hour of delay in administration of antibiotics may lead to a mean decrease in survival.3 From there, multiple studies have investigated vasopressor use, corticosteroids, and even vitamin C in the establishment of an effective set of guidelines in the management of severe sepsis and septic shock. The predominant entity regulating the policies regarding sepsis management is the Surviving Sepsis Campaign (SSC). Secondary entities, such as Centers for Medicare and Medicaid Services, require sepsis bundle timeline cut-offs for a health care entity to receive reimbursement. Despite many years of sepsis research, physicians still receive continuous updates suggesting new strategies to increase overall survival and decrease mortality in this patient population. A summary of these recommendations can be found in Table 1.
Explore This Issue
ACEP Now: Vol 43 – No 06 – June 2024What Is “Sepsis”?
In sepsis, a pathogen triggers an initial exaggerated inflammatory-immune response that leads to activation or suppression of multiple pathways, in turn leading to circulatory and metabolic dysfunction.4 The clinical definition of sepsis is the recognition of two SIRS criteria with evidence of infection. Severe sepsis is defined as sepsis with evidence of organ dysfunction. One result of this dysregulated response is hemodynamic instability despite fluid resuscitation, ultimately classified as “septic shock,” which persistently remains a significant cause of mortality.5
What Can I Do to Combat Sepsis in 2024?
1. Screening tools
Multiple screening tools in the emergency department (ED) have been studied, including qSOFA, SIRS, NEWS, and MEWS. Sepsis-3 first considered the qSOFA score to be the superior screening tool to utilize in early sepsis identification.6 The SSC guidelines for 2021 recommend against the use of qSOFA when compared to SIRS, NEWS, or MEWS.7 A more recent study compared SIRS (body temperature above 38°C or below 36°C, heart rate greater than 90 beats per minute, respiratory rate greater than 20 breaths per minute, neutrophilia above 12,000 mm3 or below 4000 mm3) and qSOFA (systolic blood pressure <100 mmHg, respiratory rate >22, and Glasgow coma scale <15) to a Sepsis Prediction Model, which generates a sepsis score based on electronic health record-confirmed sepsis; however, its application in the clinical setting was limited by poor timeliness in comparison to SIRS and qSOFA.8
In summary, qSOFA should not be used as a single screening tool for sepsis. NEWS, MEWS, or SIRS may be more beneficial at this time.
2. Lactate clearance and capillary refill time (CRT)
Most institutions are beginning to transition to electronic health record-generated order sets for sepsis when it is suspected by the practitioner; however, the “necessary” labs to obtain have varied by clinical center. Specifically, lactate clearance has been a debatable topic since its inception. The SSC guidelines for 2021 weakly recommended measuring blood lactate and, if elevated (defined as ≥4 mmol/L), guiding resuscitation by a decrease in its value over time.7 The ANDROMEDA-SHOCK study evaluated whether targeting resuscitation towards a CRT versus lactate clearance led to a reduced mortality at 28 days; ultimately, there was no detectable difference. The CRT group’s mortality rate was 34.9 percent versus 43.4 percent in the lactate group.9 A concern raised with both strategies was whether volume overload and its early recognition was key to preventing further complications as a result of over-resuscitation. ACEP’s own clinical policy does not recommend the use of serum lactate levels to guide ongoing IV fluid resuscitation, as it has not been found to be an accurate marker of ongoing fluid needs.10
In summary, there is no good “endpoint” guiding resuscitation in septic shock, but CRT or lactate clearance can be considered.
3. Intravenous fluids
One of the greatest controversies in the care of sepsis has been intravenous fluid management during the acute stages of septic shock. For patients in septic shock or exhibiting signs of hypoperfusion, SSC guidelines weakly recommend at least 30 mL/kg of intravenous crystalloid within the first three hours of resuscitation.7 The current fluid recommendation is a balanced crystalloid, with lactated ringers being superior to normal saline.5 The CLOVERS trial investigated conventional fluid resuscitation versus early vasopressor use and intravenous fluid restriction, with the conventional group receiving approximately 3.8 L and the fluid restriction group receiving 1.8 L. Researchers concluded that at 90 days, mortality and adverse events of the aforementioned volumes were similar between the two groups.11 Notably, ACEP’s clinical policy does not endorse an empirical fluid bolus but, rather, individualized fluid resuscitation needs for each patient.10 The judicious amount of intravenous fluids cited by the SSC should also be liberalized for patients with clinical findings of volume overload or a known reduced ejection fraction.
In summary, the current recommendation is 30 mL/kg of lactated ringers within the first three hours of resuscitation, with the caveat of utilizing clinical judgment to prevent fluid overload.
4. Antibiotics
Empiric broad-spectrum antibiotic regimens in the acute stages of severe sepsis and septic shock are becoming a mainstay of treatment. The SSC guidelines base antimicrobial administration recommendations on the following: likelihood of a current infectious process based on clinical examination, suspicion for MRSA or MDR organisms, and viral or fungal sources.7 MRSA nasal swabs, when negative, can eliminate the need for MRSA coverage in sepsis patients.5 There is no current recommendation for a specific antibiotic regimen, but administration of broad-spectrum coverage must be completed within one hour of recognizing septic shock (strong recommendation) or within three hours of recognizing sepsis without shock.5 Each institution will have its own unique microbiome, but, as an example, a suggested antibiotic regimen is presented in tabular form based on Michigan Medicine’s recommendations (Table 2).5 Ferrer and colleagues investigated time to antibiotic administration in severe sepsis and septic shock, concluding that for every hour a physician delays antibiotic coverage, there was a statistically significant increase in mortality.12 Another retrospective cohort study investigated the association between door-to-antibiotic time of patients with clinical sepsis and longterm mortality. Their median door-to-antibiotic time averaged 166 minutes, leading to a one-year mortality risk of 19 percent. Each additional hour delay in antibiotic administration was associated with 10 percent increased odds of one-year mortality.13
In summary, the current recommendations for antibiotic coverage are based on suspicion for an acute bacterial process, and administration of a “broad” antimicrobial regimen is recommended within one hour of recognizing septic shock or within three hours in sepsis without shock.
5. Vasopressors
Current recommendations by the SSC follow a stepwise pattern when considering vasopressor therapy after adequate volume resuscitation. The first recommendation is the use of norepinephrine, followed by vasopressin and then epinephrine if a MAP >65 mmHg cannot be obtained. If there is cardiac dysfunction, one may add dobutamine to supplement norepinephrine or utilize epinephrine alone.7 It is important to mention that both the CENSER and CLOVERS trials investigated standard sepsis management (intravenous fluids followed by vasopressors) versus the early use of norepinephrine. CENSER determined that early norepinephrine use was associated with increased shock control by six hours compared to standard therapy, and CLOVERS concluded that mortality and adverse events were similar between the two groups.11,14 For adults with septic shock on norepinephrine with inadequate MAP levels (<65 mmHg), vasopressin is recommended rather than escalating doses of norepinephrine.5
In summary, the current recommendations for vasopressor use in severe sepsis and septic shock follow an algorithmic approach, titrating a MAP >65 mmHg: intravenous fluid bolus, followed by norepinephrine, vasopressin, and then epinephrine if there is no underlying cardiac dysfunction.
6. Steroids
Another controversial topic in sepsis management has been the use of corticosteroids. The current recommendation by the SSC is the use of intravenous corticosteroids with ongoing vasopressor therapy.7 One regimen includes hydrocortisone 200 mg/day, 50 mg every six hours or as a continuous infusion.15 Interestingly, the HYPRESS trial studied the early use of hydrocortisone therapy in patients with severe sepsis who had not yet developed septic shock. They concluded that hydrocortisone compared with placebo did not reduce the risk of septic shock within 14 days.16
The current recommendation is the use of corticosteroids with ongoing vasopressor therapy or hypotension despite vasopressors.
7. Random facts
Vitamin C
A study by Lamontagne and colleagues found that in adults with sepsis currently receiving vasopressor therapy in the ICU, administration of intravenous vitamin C had a higher risk of death at 28 days compared to placebo.17
The current recommendation from the SSC suggests against intravenous vitamin C.7
Albumin
The ALBIOS study investigated albumin replacement in addition to crystalloids compared to crystalloids alone in overall survival of patients with severe sepsis. They concluded that the addition of albumin did not improve overall survival at 28 and 90 days.18
The current recommendation from the SSC suggests utilizing albumin in patients who have received large volumes of crystalloid.7
Protein C
The PROWESS-SHOCK trial compared recombinant human activated protein C versus placebo and concluded there was no significant reduction in mortality at 28 or 90 days compared to placebo.19
There are no current recommendations from the SSC to use activated protein C.
ECMO
A study conducted by Helwani and colleagues demonstrated the use of VA-ECMO in patients with sepsis-induced cardiomyopathy. In the setting of persistent hypotension despite standard management of septic shock with evidence of severe cardiac systolic dysfunction and end-organ perfusion, VA-ECMO should be considered.20
Current recommendations by the SSC are the utilization of VV-ECMO in the setting of severe acute respiratory distress syndrome when conventional mechanical ventilation fails, without mention of VA-ECMO.7
 Dr. Carvey is a resident at MetroHealth/Cleveland Clinic Residency Emergency Medicine.
Dr. Carvey is a resident at MetroHealth/Cleveland Clinic Residency Emergency Medicine.
 Dr. Glauser is an emergency physician at MetroHealth Medical Center, Cleveland, Ohio and professor of emergency medicine at Case Western Reserve University.
Dr. Glauser is an emergency physician at MetroHealth Medical Center, Cleveland, Ohio and professor of emergency medicine at Case Western Reserve University.
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34(2):77-84.
- King J, Chenoweth CE, England PC, et al. Early Recognition and Initial Management of Sepsis in Adult Patients [Internet]. Ann Arbor, Mich.: Michigan Medicine University of Michigan; 2023. https://www.ncbi.nlm.nih.gov/books/NBK598311/.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-810.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11)e1063-e1143.
- Schertz AR, Lenoir KM, Bertoni AG, et al. Sepsis prediction model for determining sepsis vs SIRS, qSOFA, and SOFA. JAMA Netw Open. 2023;6(8):e2329729.
- Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654-664.
- Spiegel R, Farkas JD, Rola P, et al. The 2018 surviving sepsis campaign’s treatment bundle: when guidelines outpace the evidence supporting their use. Ann Emerg Med. 2019;73(4):356-358.
- Shapiro NI, Douglas IS, Brower RG, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension (CLOVERS). N Engl J Med. 2023;388(6):499-510.
- Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749-1755.
- Peltan ID, Brown SM, Bledsoe JR, et al. ED door-to-antibiotic time and long-term mortality in sepsis. Chest. 2019;155(5):938-946.
- Permpikul C, Tongyoo S, Viarasilpa T, et al. Early use of norepinephrine in septic shock resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med. 2019;199(9):1097-1105.
- Srzić I, Nesek Adam V, Tunjić Pejak D. Sepsis definition: What‘s new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl 1):67-72.
- Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis: The HYPRESS randomized clinical trial. JAMA. 2016;316(17):1775-1785.
- Lamontagne F, Masse MH, Menard J, et al. Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med. 2022;386(25):2387-2398.
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412-1421.
- Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064.
- Helwani MA, Lim A. Is venoarterial extracorporeal membrane oxygenation an option for managing septic shock. Curr Opin Anaesthesiol. 2023;36(1):45-49.






One Response to “Updates in the Management of Severe Sepsis and Septic Shock”
July 1, 2024
Joseph R Shiber, MDDear ACEPNow Editor,
Excellent synopsis of ED treatment of septic shock but I would like to add a few clarifications. The preferred balanced IVF is Plasmalyte-A since LR is somewhat hypotonic (Na 130) and uses lactate as a buffer, compared to acetate and gluconate in Plasmalyte-A (Na 140). The additional lactate is not actually detrimental to cellular activity but can hamper the usefulness of tracking lactate levels especially with hepatic or mitochondrial dysfunction where lactate is not being converted back to pyruvate for preparation to enter the TCA cycle. The optimal vasopressor for septic shock should correct the hemodynamic disorder(s) causing the tissue hypoxia. Levophed is certainly the most useful to help restore vascular tone (alpha effect) in the low SVR vasodilatory state of distributive shock while supplying a small B1-2 effect but there are cases where an inappropriate heart-rate response occurs (HR <80) due to medications (such as AVN blockers) or to intrinsic chronotropic failure (age or sepsis related). In these cases, it is paramount to address the heart rate at the same time, since if the heart rate remains inappropriately low while simply increasing SVR the cardiac output and tissue perfusion will potentially go down not up. Lastly, although ECMO is well recognized as a rescue for ARDS (V-V) and circulatory shock (V-A) it should be noted that active bacteremia or fungemia is a contraindication since the circuit will be contaminated immediately and cannot be sterilized.
Respectfully,
Joseph Shiber, MD, FACEP, FACP, FNCS, FCCM