The Case
A 28-year-old woman presents with nausea and vomiting during pregnancy. She is at eight weeks’ gestation and frustrated that nothing has worked. She has tried ginger, acupressure, vitamin B6 with doxylamine, and dimenhydrinate. A friend in her prenatal class told her about ondansetron, but a quick Google search mentioned birth defects, and this scared her. She wants to know what else she could safely try.
Explore This Issue
ACEP Now: Vol 38 – No 09 – September 2019Background
Nausea and vomiting in pregnancy is a very common and frustrating condition. In more than 30 percent of women, the symptoms can become clinically significant. The most common cause of hospitalization in early pregnancy is hyperemesis gravidarum.
The American College of Obstetricians and Gynecologists has published an algorithm for nausea and vomiting in pregnancy in a practice guideline (see Figure 1).1 It starts with nonpharmacological options like acupressure with wrist bands (despite evidence of efficacy). Pharmacological options include vitamin B6 alone or in combination with doxylamine. The next step is adding dimenhydrinate, prochlorperazine, or promethazine. The algorithm then dichotomizes into no dehydration or dehydration with persistent symptoms. It is at this step when ondansetron is added as a possible treatment.
The evidence of fetal safety of ondansetron is conflicting. An observational study showed that ondansetron taken during pregnancy was not associated with an important increased risk of fetal harm.2
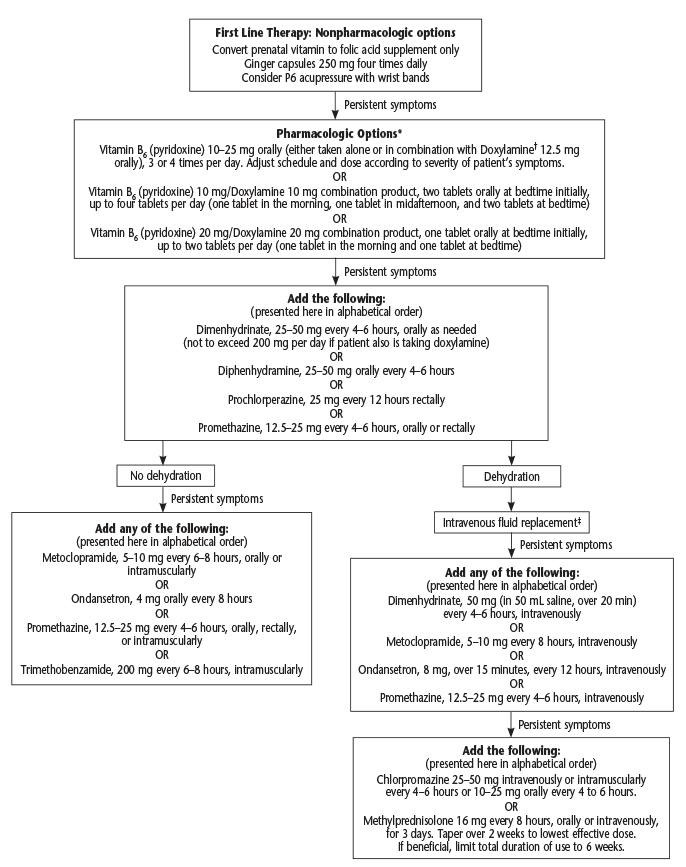
(click for larger image) Figure 1: Algorithm for Nausea and Vomiting of Pregnancy
Source: Obstet Gynecol. 2018;131(1):190-193. Reprinted with permission.
Clinical Question
Is the use of ondansetron during pregnancy associated with congenital malformations?
The Study
Huybrechts KF, Hernández-Díaz S, Straub L, et al. Association of maternal first-trimester ondansetron use with cardiac malformations and oral clefts in offspring. JAMA. 2018;320(23):2429-2437.
- Population: Women ages 12 to 55 years on Medicaid from three months prior to conception to one month postpartum.
- Exposure: Women who were pregnant and filled at least one prescription for ondansetron during the first three months (12 weeks) of pregnancy.
- Excluded: Women who filled a prescription during the three months before the start of their pregnancy.
- Comparison: Woman who were pregnant and filled a prescription for pyridoxine, promethazine, metoclopramide, or any alternative treatments.
- Outcome:
- Primary Outcomes: Cardiac malformations and oral clefts diagnosed within 90 days after delivery.
- Secondary Outcomes: Subgroups of cardiac malformations and oral clefts evaluated along with congenital malformations overall.
Authors’ Conclusions
“Among offspring of mothers enrolled in Medicaid, first-trimester exposure to ondansetron was not associated with cardiac malformations or congenital malformations overall after accounting for measured confounders but was associated with a small increased risk of oral clefts.”
Key Results: This observational study included 1.5 million women and 1.8 million pregnancies. The mean age was 24 years, and 5 percent were potentially exposed to ondansetron in the first trimester.
No increased risk of cardiac malformation was observed, but there was a statistical increase in the risk of oral clefts.
- Primary Outcomes:
- Cardiac malformation: adjusted relative risk (RR) 0.99 (95% CI, 0.93–1.06)
- Oral clefts: adjusted RR 1.24 (95% CI, 1.03–1.48)
- Secondary Outcome: Overall malformations: adjusted RR 1.01 (95% CI, 0.98–1.05)
Evidence-Based Medicine Commentary
- Observational Study: The largest limitation to this study is the observational design. It demonstrates associations, not causation. Other confounders and co-variates could have been responsible for any difference discovered in the dataset with ondansetron exposure during pregnancy.
- Exposed Women: The exposed pregnant women had different baseline characteristics than those women not exposed to ondansetron. Exposed pregnant women were more likely to smoke; have psychiatric diagnosis or neurologic condition; be white; and fill a prescription for other nausea and vomiting medication, psychotropics, steroids, and suspected teratogens. These differences could have affected the results.
- External Validity: This dataset captured 1.5 million women and 1.8 million pregnancies. This is a large number but only represents 50 percent of all the pregnancies in the United States. The pregnant women included were Medicaid patients and may be different than pregnant women with other insurance types.
Bottom Line
Ondansetron exposure in early pregnancy does not appear to be associated with an overall increased risk of fetal malformations, but there may be a small statistical increased risk of oral clefts.
Case Resolution
You advise her that metoclopramide is recommended to be used before ondansetron. She responds well to a 5 mg oral dose in the emergency department. She is discharged home with a prescription for metoclopramide. You advise her to return if her nausea and vomiting do not improve, she is unable to tolerate oral intake, or she is losing weight.
Thank you to Dr. Nick Papalia who is currently completing his obstetrics and gynecology residency at the University of Calgary in Alberta, Canada.
Remember to be skeptical of anything you learn, even if you heard it on the Skeptics’ Guide to Emergency Medicine.
References
- ACOG Practice Bulletin No. 189 summary: nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131(1):190-193.
- Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368(9):814-823.
Pages: 1 2 | Multi-Page





One Response to “What’s the Best Option for Queasiness During Pregnancy?”
September 22, 2021
FrankFowerPeriactin / Cyproheptadine 4 mg ,po, BID
I have Tried All your mentioned Algorithms,
During my practice for 45 years…
The one and only one that consistently works and loved by patients and Never had any issues is Periactin