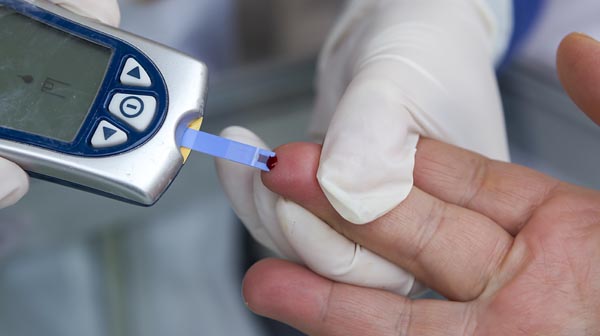
Rapid testing is the way of the future, and it begins with point-of-care testing (POCT). POCT moves testing from the laboratory setting to the patient’s bedside, which has had a positive impact on therapeutic turnaround time. Rapid testing gives accurate results in hours, not days, and in some cases, results are available in minutes.1 Currently, POCT is available for whole blood profile, cardiac markers, drug screens, basic metabolic panel, HIV, hepatitis C virus (HCV), syphilis, stroke, cancer, strep infection, and influenza.2
As the director of patient care in the emergency department (ED), emergency physicians play an important role in the POCT process. This includes implementing policies and procedures that will improve patient care and control costs. POCT requires that new policies and procedure be evaluated and implemented and will have an effect on cost.
With POCT, test results are returned rapidly and testing costs are greatly diminished. Rapid tests, such as those for HIV and HCV, cost between $10 and $30 per test kit, while laboratory testing may cost several hundreds of dollars and take several days. Additionally, the cost of POCT testing can be a fee generated for the ED, and in the current world of contracted laboratory services, become a revenue generator.
Studies have shown that rapid testing has decreased the number of antiretroviral postexposure prophylaxis regimens that were started while waiting for source patient test results. This clearly is a cost benefit for the health care provider.
Origin of Rapid Testing
The Centers for Disease Control and Prevention (CDC) guidelines for postexposure prophylaxis for hepatitis B virus (HBV), HCV, and HIV are well recognized as a standard of medical care, and are enforced by the U.S. Department of Labor’s Occupational Safety & Health Administration (OSHA), as noted in OSHA’s Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens.3,4
In June 2001, the CDC updated its guidelines for postexposure source patient testing, to recommend that “testing to determine the HBV, HCV, and HIV infection status of an exposure source should be performed as soon as possible. Hospitals, clinics, and other sites that manage exposed [health care personnel] HCP should consult their laboratories regarding the most appropriate test to use to expedite obtaining these results. An FDA-approved rapid HIV-antibody test kit should be considered for use in this situation, particularly if testing by [enzyme immunoassay] EIA cannot be completed within 24–48 hours.”3
This was the beginning of rapid testing related to postexposure medical follow-up. Rapid testing for HIV has been available since 1998, and in 2010 the FDA approved rapid testing for HCV. It is important to note that rapid HIV and rapid HCV testing has been waived from Clinical Laboratory Improvement Amendments (CLIA) compliance and can be done in the ED.5,6 In fact, rapid HIV testing was piloted in EDs. Because the current rapid HIV tests screen for proteins present in the beginning the life cycle of the virus, the CDC has published that there is no window phase with rapid HIV testing.7
In 2003, the CDC and World Health Organization (WHO) published guidelines for POCT for HIV.8 It was determined that rapid HIV testing would serve to assist in determining if antiretroviral drugs were appropriate following an occupational exposure. In 2006, the CDC requested that there be universal screening for HIV in populations with a prevalence of undiagnosed infections greater than 0.1 percent.9 Approximately 16 states passed legislation in support of this request, however, very few medical facilities or practices have implemented the process. This CDC request was adopted by ACEP in 2011, but also has not been widely followed in practice.
Testing for HCV, Syphilis
In May 2013, the CDC published guidelines for HCV testing for clinicians and laboratorians stating the need to identify, through testing, people with current HCV infections. These guidelines note that rapid testing for HCV antibodies can be used for screening. There is also a focus on screening all persons born between 1945 and 1965. New York State passed a law effective as of January 1, 2014 which requires every person born between 1945 and 1965 to be offered an HCV screening test during inpatient hospital care or primary care, in keeping with the CDC’s goal.10,11
Syphilis testing may also be indicated. Testing for syphilis postexposure is recommended if the source is HIV-positive as many persons are co-infected with syphilis. In 2010 the CDC published guidelines on curbing sexually transmitted diseases in which it noted that “universal screening should be considered on the basis of the local area and institutional prevalence of early (primary, secondary, and early latent) infectious syphilis.”12 At least 18 states have passed plans to reduce syphilis cases.12
Rapid HCV testing is of great value in postexposure source patient testing because it can bring closure to health care workers’ concerns. It should be noted that if a rapid HCV test is positive, confirmatory testing for viral load (HCV-RNA) is to be performed. This also benefits the HCV-positive patient by enabling them to be referred for proper medical treatment. Most cases of HCV can now be cured.
Putting into place POCT and rapid testing would identify persons who may be unaware of their disease status and offer initial treatment and/or proper referral. POCT and rapid testing would assist in expediting proper follow-up for the exposed health care worker if the source is positive.
References
- Dilts TJ. Point-of-care testing: a step to the future. Franklin Lakes, NJ. Becton-Dickinson Vacutainer Lab Notes. 2013;8(1): 1-13.
- London, S. Point-of-care testing useful when appropriate. ACEP News. http://acep.org/Content.aspx?id=79745. Published February 2011. Accessed August 20, 2014.
- Centers for Medicare & Medicaid Services. Clinical laboratory improvement amendments (CLIA). www.cms.hhs.gov/CLIA. Published February 27 2014. Accessed August 20, 2014.
- U.S. Food and Drug Administration. CLIA waiver granted to OraQuick ADVANCE Rapid HIV-1/2 antibody test. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/OrthopaedicandRehabilitationDevicesPanel/ucm125023.htm. Published August 1, 2014. Accessed October 2, 2014.
- Kuhar DT, Henderson DK, Strubble KA, et al. Updated US public health service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiolog. 2013; 34(9):875-892.
- Centers for Disease Control and Prevention. Rapid point-of-service testing: A public health perspective. https://wwwn.cdc.gov/mlp/pdf/KeystonePOCCrev.pdf. Published September 17, 2003. Accessed August 20, 2014.
- Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
- New York State Department of Health. NYS hepatitis C testing. http://www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/rapid_antibody_testing/. Published February 2014. Accessed August 20, 2014.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. http://www.cdc.gov/std/treatment/2010/. Published December 16, 2010. Updated April 17, 2014. Accessed August 20, 2014.
- Centers for Disease Control and Prevention. Updated US public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Published July 2, 2001. Accessed August 20, 2014.
- U.S. Department of Labor Occupational Safety & Health Administration. Enforcement procedures for the occupational exposure to bloodborne pathogens. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=directives&p_id=2570. Published November 27, 2001. Accessed August 20, 2014.
- Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. Published September 22, 2006. Accessed August 20, 2014.
Ms. West is an infection control consultant at Infection Control/Emerging Concepts, Inc.
Pages: 1 2 3 | Multi-Page




No Responses to “Point-of-Care Testing and Postexposure Prophylaxis: The Emergency Physician’s Role”